Why does this structural formula not represent an actual molecule? | |
Question:
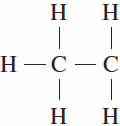
Transcribed Image Text:
Н Н —С — С | | Н Н Н
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (14 reviews)
The rightha...View the full answer

Answered By

Muhammad Salman Alvi
Well, I am a student of Electrical Engineeing from Information Technology University of Punjab. Just getting into my final year. I have always been good at doing Mathematics, Physics, hardware and technical subjects. Teaching profession requires a alot of responsibilities and challenges.
My teaching experience started as an home tutor a year ago. When I started teaching mathematics and physic subjects to an O Level student. He was about 14 years old. His name was Ibrahim and I used to teach him for about 2 hours daily. Teaching him required a lot of patience but I had to be polite with him. I used to give him a 5 min break after 1 hour session. He was quite weak in basic maths and calculation. He used to do quite a lot of mistakes in his homework which I gave him weekly. So I decided to teach him basics from scratch. He used to say that he got the concept even if he didn't. So I had to ask him again and again. I worked on his basics for a month and after that I started taking a weekly test sesions. After few months he started to improve gradually. Now after teaching him for about a year I can proudly say that he has improved alot. The most important thing was he managed to communicate all the difficullties he was facing. He was quite capable and patient. I had a sincere desire to help him reach to its full potential. So I managed to do that. We had a very good honest relationship of a student and a teacher. I loved teaching him as a tutor. Now having an experience of one year teaching I can read students quite well. I look forward to work as an online tutor who could help students in solving their all sort of difficulties, problems and queries.
4.90+
29+ Reviews
43+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Consider the molecule CH3CH2CH=CHCH3. a. Write a structural formula of the cis isomer. b. Name the molecule from part a. c. Write the condensed structural formula of the major product of the reaction...
-
The generic structural formula for a 1-alkyl-3-methylimidazolium cation is Where R is a ¬CH2(CH2)nCH3 alkyl group. The melting points of the salts that form between the...
-
The structural formula for the linear form of D-mannose is (a) How many chiral carbons are present in the molecule? (b) Draw the structure of the six-member-ring form of this sugar. CH -- -- H-C-OH...
-
Maicom Construction Materials Inc. , hereinafter referred to as "MCM", is a construction materials company established in Moncton, New Brunswick. Its facilities (warehouse, store and offices) are...
-
Are there some ethical values or principles that you believe are relative to one's own culture, religion, or personal opinion? Are there some that you believe are not? what makes them different?
-
Describe the major sources of error related to fieldwork.
-
What are four principles of effective postproject reviews?
-
Kaelea, Inc., has no debt outstanding and a total market value of $70,000. Earnings before interest and taxes, EBIT, are projected to be $6,000 if economic conditions are normal. If there is strong...
-
On January 1, a company issues bonds dated January 1 with a par value of $350.000. The bonds mature in 5 years. The contract rate is 7%, and interest is paid semiannually on June 30 and December 31....
-
Aaron, Deanne, and Keon formed the Blue Bell General Partnership at the beginning of the current year. Aaron and Deanne each contributed $110,000 and Keon transferred an acre of undeveloped land to...
-
In which of the compounds C 2 H 2 , C 2 H 4 , and C 4 H 10 are the carbon-carbon bonds single, in which are they double, and in which are they triple?
-
In general, how do the reactivities of hydrocarbon molecules that contain only single bonds compare with the reactivities of hydrocarbon molecules that contain double or triple bonds as well?
-
A ball is released from rest relative to the elevator at a distance h 1 above the floor. The speed of the elevator at the time of ball release is v 0 . Determine the bounce height h 2 of the ball (a)...
-
How do you manage global and international teams? What would you do different?
-
Your friend is super excited about the results of their study! They examined whether different parenting styles [A] resulted in differences in anxiety levels among children. The different levels (a)...
-
Test the series for convergence or divergence. n=1 e1/n 78 O convergent O divergent
-
How do employees perceive the organization's vision and mission, and to what extent do these perceptions influence their commitment to the organization ?
-
In the table below which shows class taken and grade achieved, find the probability that a student selected takes Stat or receives a B grade. Round your answer to three decimal places 40 70 70 50 40...
-
Solve equation. 2 |x| = 8
-
U.S. households have become smaller over the years. The following table from the 2010 GSS contains information on the number of people currently aged 18 years or older living in a respondent's...
-
The parameter a in the van der Waals equation is greater for H 2 O than for He. What does this say about the difference in the form of the potential function in Figure 1.10 for the two gases? Figure...
-
Compound A and compound B are constitutional isomers with molecular formula C 4 H 9 Cl. Treatment of compound A with sodium methoxide gives trans-2-butene as the major product, while treatment of...
-
An unknown compound with molecular formula C 6 H 13 Cl is treated with sodium ethoxide to produce 2,3-dimethyl-2-butene as the major product. Identify the structure of the unknown compound.
-
XF Ltd. Is an expanding private company in the electric trade. Accounts preparing in January 2019 included the following information: Profit Statement for the year ended 31 st December 2018 Kshs.000...
-
Check On June 15, 2021, Sanderson Construction entered into a long-term construction contract to build a baseball stadium in Washington D.C., for $340 million. The expected completion date is April...
-
Q.1 Bassem Company purchased OMR420,000 in merchandise on account during the month of April, and merchandise costing OMR $350,000 was sold on account for OMR 425,000. Required: 1. Prepare journal...

Study smarter with the SolutionInn App


