Question: Compute an upper bound on the energy density of a lead-acid battery in which the overall chemical process is ? and for each such reaction
Compute an upper bound on the energy density of a lead-acid battery in which the overall chemical process is
?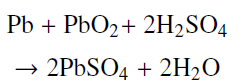
and for each such reaction two electrons pass around the external circuit, assuming that the only material needed is that involved in the reaction. The standard energy density is significantly lower than the upper bound due to the need for additional water, as well as other battery components, for operation of the battery.
Pb + PbO2+ 2H2SO4 2PBSO4 + 2H2O
Step by Step Solution
3.39 Rating (168 Votes )
There are 3 Steps involved in it
From eq 377 G 0 nF E 0 cell 29648 ... View full answer

Get step-by-step solutions from verified subject matter experts


