A few drops of each of the indicators shown in the accompanying table were placed in separate
Question:
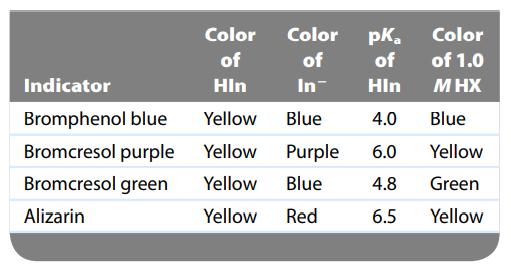
A few drops of each of the indicators shown in the accompanying table were placed in separate portions of a 1.0-M solution of a weak acid, HX. The results are shown in the last column of the table. What is the approximate pH of the solution containing HX? Calculate the approximate value of Ka for HX.
Transcribed Image Text:
Color Color pka of of of Indicator Hin In Hin Bromphenol blue Yellow Blue 4.0 Bromcresol purple Yellow Purple 6.0 Bromcresol green Yellow Blue 4.8 Alizarin Yellow Red 6.5 Color of 1.0 M HX Blue Yellow Green Yellow
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (12 reviews)
Solution a The approximate pH of the solution contai...View the full answer

Answered By

James Warinda
Hi! I’m James Otieno and I'm an experienced professional online tutor with countless hours of success in tutoring many subjects in different disciplines. Specifically, I have handled general management and general business as a tutor in Chegg, Help in Homework and Trans tutor accounts.
I believe that my experience has made me the perfect tutor for students of all ages, so I'm confident I can help you too with finding the solution to your problems. In addition, my approach is compatible with most educational methods and philosophies which means it will be easy for you to find a way in which we can work on things together. In addition, my long experience in the educational field has allowed me to develop a unique approach that is both productive and enjoyable.
I have tutored in course hero for quite some time and was among the top tutors awarded having high helpful rates and reviews. In addition, I have also been lucky enough to be nominated a finalist for the 2nd annual course hero award and the best tutor of the month in may 2022.
I will make sure that any student of yours will have an amazing time at learning with me, because I really care about helping people achieve their goals so if you don't have any worries or concerns whatsoever you should place your trust on me and let me help you get every single thing that you're looking for and more.
In my experience, I have observed that students tend to reach their potential in academics very easily when they are tutored by someone who is extremely dedicated to their academic career not just as a businessman but as a human being in general.
I have successfully tutored many students from different grades and from all sorts of backgrounds, so I'm confident I can help anyone find the solution to their problems and achieve
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
The data shown in the accompanying table were obtained from a tensile test of high-strength steel. The test specimen had a diameter of 0.505 in. and a gage length of 2.00 in. (see figure for Prob....
-
The observations shown in the accompanying table were drawn from a randomized block design with no interaction. a. Calculate SST, SSA, SSB, and SSE. b. Calculate MSA, MSB, and MSE. c. Construct an...
-
A 0.01 M solution of a weak acid in water is 0.05% ionized at 25C. What is its pK?
-
A steam generator consists of a bank of stainless steel (k = 15 W/m K) tubes having the core configuration of figure and an inner diameter of 13.8 mm. The tubes are installed in a plenum whose...
-
Two different companies are considering rights offerings. The current market price per share is $48 in both cases. To allow for fluctuations in market price, company X wants to set a subscription...
-
Nonzero initial velocity is more of theoretical interest because it is difficult to obtain experimentally. Show that for (17) to satisfy (9b) we must have where K m = 2/(cα m R)J 2 1...
-
How do you think a jury would respond to this situation? What level of damages, if any, do you think a jury would be included to award in this case?
-
This case continues the financial statement analysis of Procter& Gamble Co. begun in Mini case 9.1 and developed further in Mini case 11.1. This final installment covers issues in dealing with core...
-
4. Using the statistics in the following report generated for Community Hospital, calculate (round to two decimal places) the percentage of occupancy for the month of December. Remember to calculate...
-
Jia Inc. applies ASPE and had the following statement of financial position at the end of operations for 2013: During 2014, the following occurred: 1. Jia Inc. sold some of its fair value-net income...
-
A 10.00-g sample of the ionic compound NaA, where A - is the anion of a weak acid, was dissolved in enough water to make 100.0 mL of solution and was then titrated with 0.100 M HCl. After 500.0 mL...
-
A buffer is made using 45.0 mL of 0.750 M HC 3 H 5 O 2 (K a = 1.3 10 -5 ) and 55.0 mL of 0.700 M NaC 3 H 5 O 2 . What volume of 0.10 M NaOH must be added to change the pH of the original buffer...
-
The adjusting entry for unearned revenue should a. debit the expenses account and credit the asset account. b. debit the expenses account and credit the liability account. c. debit the asset account...
-
You have been employed as a systems analyst in the information systems organization of a medium-sized consumer goods manufacturer for three years. You are quite surprised when your manager offers you...
-
For your initial post, address the following: First, introduce yourself to the class by sharing a bit about yourself, such as your preferred name or pronouns, where you are from, what your major is,...
-
Question 8 : Consider the technology of Solar Panels. Which stage of the technology life cycle S curve is this technology in. Justify why ? Question 9 : The standard Product Life Cycle has 5 stages...
-
At Benihana restaurant a man wrenched his neck while ducking a piece of flying shrimp, requiring treatment by several doctors. By that summer, doctors determined surgery was necessary to treat...
-
You have just come into an inheritance of $25,000 from a distant relative, and you want to invest it for the long term. Provide an investment portfolio that includes five different stocks. Report the...
-
Use the information given about the angle , 0 2 to find the exact value of (a) sin(2) (b) cos(2) (c) sin /2 (d) cos /2 3 0 < 0 < - 5' TT sin 0 2
-
What is taxable income, and what is the formula for determining taxable income?
-
How might you replace a halogen substituent by a deuterium atom if you wanted to prepare a deuteratedcompound? Br , CHH-H CHCH2CH
-
How would you carry out the following transformations using an organo copper coupling reaction? More than one step is required in eachcase. (a) "CH (b) HH2CH2CHBr CH3CH2CH2CH2CH2CH2CH2CH3 (c)...
-
Rank each of the following series of compounds in order of increasing oxidationlevel: CI (a) (b) CH3CN CH3CH2NH2 H2NCH2CH2NH2
-
You are thinking of buying a stock priced at $99 per share. Assume that the risk-free rate is about 4.5% and the market risk premium is 6.4%. If you think the stock will rise to $125 per share by the...
-
The transactions in this practice set were completed by Hydro Paddle Boards, Inc. during January, the first month of the companys fiscal year. Hydro Paddle Boards, Inc. is a manufacturing corporation...
-
Al preparar el estado de resultados pro forma, cules de las siguientes partidas se deducen de las utilidades brutas para llegar a las ganancias despus de impuestos? Pregunta de seleccin mltiple....

Study smarter with the SolutionInn App


