a. Name each of the following alcohols. b. Name each of the following alcohols, including the stereochemistry
Question:
a. Name each of the following alcohols.
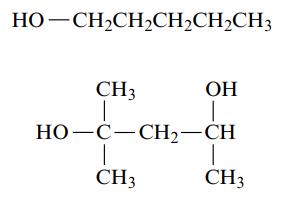
b. Name each of the following alcohols, including the stereochemistry if cis–trans isomers are possible.
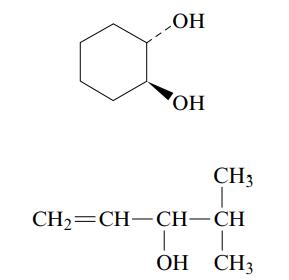
Transcribed Image Text:
HỌ—CH,CH,CH,CH,CH3 ОН I CH3 T HỌ–C–CH2–CH 1 CH3 CH3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (7 reviews)
ANSWER Alcohols are substances in which any carbon atom in the main carbon chain has a hydroxyl grou...View the full answer

Answered By

Aketch Cindy Sunday
I am a certified tutor with over two years of experience tutoring . I have a passion for helping students learn and grow, and I firmly believe that every student has the potential to be successful. I have a wide range of experience working with students of all ages and abilities, and I am confident that I can help students succeed in school.
I have experience working with students who have a wide range of abilities. I have also worked with gifted and talented students, and I am familiar with a variety of enrichment and acceleration strategies.
I am a patient and supportive tutor who is dedicated to helping my students reach their full potential. Thank you for your time and consideration.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
Each of the following alcohols has been subjected to acidcatalyzed dehydration and yields a mixture of two isomeric alkenes. Identify the two alkenes in each case, and predict which one is the major...
-
Each of the following alcohols has been prepared by reaction of a Grignard reagent with ethylene oxide. Select the appropriate Grignard reagent in each case. (a) CH3 CH2CH2OH CH2CH20
-
Each of the following alcohols has been subjected to acidcatalyzed dehydration and yields a mixture of two isomeric alkenes. Identify the two alkenes in each case, and predict which one is the major...
-
Bruno Corp. has decided to expand its operations. The bookkeeper recently completed the following statement of financial position in order to obtain additional funds for expansion: Instructions (a)...
-
The Hughes Supply Company uses an inventory management method to determine the monthly demands for various products. The demand values for the last 12 months of each product have been recorded and...
-
In problem, solve each inequality. Graph the solution set. 2 6 2 1
-
Does the straight-line or effective interest method produce an interest expense allocation that yields a constant rate of interest over a bonds life? Explain.AppendixLO1
-
The unaudited income statement and balance sheet of Gourmet Foods Corporation for the years 2012 and 2011 are given below (in $ million): In 2012, Gourmet Foods sold its meat packing division for...
-
In April, you plan to start a new business called Eco Ebike due to the growing popularity and positive environmental impact of Ebikes. The idea is to have a small retail/warehouse space and focus on...
-
Aubrae and Tylor Williamson began operations of their furniture repair shop (Furniture Refinishers, Inc.) on January 1, 2019. The annual reporting period ends December 31. The trial balance on...
-
Estradiol is a female hormone with the following structure: How many chiral carbon atoms are in estradiol? HO CH3 OH
-
The two isomers having the formula C 2 H 6 O boil at -23 C and 78.5 C. Draw the structure of the isomer that boils at -23 C and of the isomer that boils at 78.5 C.
-
Describe the significance of the chemical shift in relation to the terms high-field and low-field.
-
Companies in the tire manufacturing business use a lot of property, plant, and equipment. Tyrell Rubber and Tire Corporation and Maxwell Rubber and Tire Manufacturing are two of the leading...
-
(7%) Problem 11: A student launches a small rocket which starts from rest at ground level. At a height h = 2.09 km, the rocket reaches a speed of v = 291 m/s. At that height, the rocket runs out of...
-
2. For the following three sets of electric field lines, what charge or charges would make such lines? Indicate their locations and type of charge (e.g. positive/negative) a.
-
What is the most important take-home point that you learned from this video? https://www.youtube.com/watch?v=nUZqvsF_Wt0 2. Policy Problems. What is onepolicy that creates inequality in the labor...
-
An employee had $20,300 in gross earnings up to September 20, 2021. She has the following information for her pay for the week ending September 27, 2021. Her employer contributes 100% toward CPP and...
-
Floridas Code of Judicial Conduct bars judges and candidates running for election to a judgeship from personally soliciting campaign contributions of a financial nature. Attorney Lanell...
-
Sue Deliveau opened a software consulting firm that immediately paid $2,000 for a computer. Was this event a transaction for the business?
-
Does the reduction of acetoacetyl ACP in step 6 occur on the Re face or the Si face of themolecule? NADPH NADP+ SACP SACP H3C Acetoacetyl ACP B-Hydroxybutyryl ACP
-
Identify the two steps in glycolysis in which ATP is produced.
-
Look at the entire glycolysis pathway and make a list of the kinds of organic reactions that take place-nucleophilic acyl substitutions, aldol reactions, E1cB reactions, and so forth.
-
What general conclusions can you draw about your companys liquidity, solvency and productivity based on your ratio calculations. Working Capital 2017 = $9,994 M 2016 = $10,673 M Current Ratio 2017 =...
-
Tami Tyler opened Tami's Creations, Incorporated, a small manufacturing company, at the beginning of the year. Getting the company through its first quarter of operations placed a considerable strain...
-
5. The current spot exchange rate is 0.95/$ and the three-month forward rate is 0.91/$. Based on your analysis of the exchange rate, you are pretty confident that the spot exchange rate will be...

Study smarter with the SolutionInn App


