Estradiol is a female hormone with the following structure: How many chiral carbon atoms are in estradiol?
Question:
Estradiol is a female hormone with the following structure:
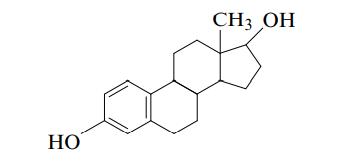
How many chiral carbon atoms are in estradiol?
Transcribed Image Text:
HO CH3 OH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 70% (10 reviews)
Estradiol is a female hormone with the following structure Water has plane of symmetry So it is ...View the full answer

Answered By

Dudhat Vaidehi
I tutored mostly elementary school students privately after school and during the summer. We met in their homes or at the public library. I charged an hourly fee, and I provided any necessary materials.
Having taught in special education in two local schools for many years meant that I had contact with a lot of parents of special needs students. I never had to advertise — word of mouth was how most folks knew of me. At one point I did have a website, but didn't utilize it much. I stayed very busy, especially in the summers, and always had a full schedule. I typically met with each student's teacher in order to get an idea of what the focus of my instruction/remediation should be. Becoming familiar with the student's learning style(s) was also very helpful. Often parents would share records and test results with me. After each tutoring session, I documented the student’s progress and gave parents written updates, as well as phone calls or emails as needed.
While my students and I certainly utilized technology and the internet often during our sessions, I never tutored online or for any tutoring company, so am not familiar with the curriculums or methods used in those settings.
Tutoring one on one was very enjoyable and rewarding. My students and I had fun, and grew quite fond of one another. The extra income was a bonus. I had to retire from tutoring due to a physically handicapping disease, and miss my students very much.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
2- A crate is supported by three cables as shown. Determine the weight of the crate knowing that the tension in cable AB is 700 lb. 46 in 50 in 33 in 30 in 70 in
-
How many chiral carbon atoms does the following structure have? ,
-
How many asymmetric carbon atoms are present in each of the following compounds? (a) (b) (c) H-C-C-C-CI H CI H OH CH CH2OH OH H H OH
-
Suppose a consumer lives in two periods , with his income in period 1 as $100 and his income in period 2 as $150. If the rate of interest in the economy is 12%. Find the equilibrium level of...
-
General American Investors Company, a closed-end regulated investment management company, invests primarily in medium- and high-quality stocks. Jim Campbell is studying the asset value per share for...
-
In problem, find the complex zeros of each polynomial function. Write f in factored form. f(x) = x 3 1
-
What factors affect the market rates for bonds?AppendixLO1
-
Telephone calls arrive at the rate of 48 per hour at the reservation desk for Regional Airways. a. Find the probability of receiving 3 calls in a 5-minute interval. b. Find the probability of...
-
Assigning Costs to Activities McCourt Company produces small engines for lawnmower producers. The accounts payable department at McCourt has 10 clerks who process and pay supplier invoices. The total...
-
You represent a Canadian toy company that's negotiating to buy miniature truck wheels from a manufacturer in Osaka, Japan. In your first meeting, you explain that your company expects to control the...
-
Ignoring ring compounds, which isomer of C 2 H 4 O 2 should boil at the lowest temperature?
-
a. Name each of the following alcohols. b. Name each of the following alcohols, including the stereochemistry if cistrans isomers are possible. HCH,CH,CH,CH,CH3 I CH3 T HCCH2CH 1 CH3 CH3
-
What should happen to a security's equilibrium interest rate as the secu- rity's liquidity risk increases? (LG4)
-
Gilbert Canned Produce (GCP) packs and sells three varieties of canned produce: green beans; sweet peas; and tomatoes. The company is currently operating at 82 percent of capacity. Worried about the...
-
Apply at least two of the theories (of your choice) to your personal experience? The theories are Leader-Member Exchange Theory (LMX Model), the Situational Leadership Model, the Contingency Model...
-
Game theory is used in economics, social science and computer science to understand and predict the behaviour of people and intelligent entities. In project management and business scenarios, it can...
-
During a chemistry lab, you take a 0.2 kg sample of ice and put it in a beaker with a thermometer. You then place the beaker with the ice on a hot plate, and turn on the hot plate. This hot plate...
-
Selected information from Carla Vista Ltd.'s statement of financial position and statement of income is as follows: Carla Vista Ltd. Statement of Financial Position (partial) December 31 2024 2023...
-
A federal law, the Immigration Reform and Control Act (IRCA) makes it unlawful for a person or other entity . . . to hire, or to recruit or refer for a fee, for employment in the United States an...
-
Refer to the data for problem 13-36 regarding Long Beach Pharmaceutical Company. Required: Compute each division's residual income for the year under each of the following assumptions about the...
-
How many molecules of acetyl CoA are produced by catabolism of the following fatty acids, and how many passages of the a-oxidation pathway arc needed? (a) Palmitic acid, CH 3 (CH 2 ) 14 CO 2 H (b)...
-
Write a mechanism for the dehydration reaction of -hydroxybutyryl ACP to yield crotonyl ACP in step 7 of fatty-acid synthesis.
-
Evidence for the role of acetate in fatty-acid biosynthesis comes from isotope-labeling experiments. If acetate labeled with 13C in the methyl group (13CH3C02H) were incorporated into fatty acids at...
-
business law A partner may actively compete with the partnership True False
-
A company provided the following data: Selling price per unit $80 Variable cost per unit $45 Total fixed costs $490,000 How many units must be sold to earn a profit of $122,500?
-
Suppose a 10-year, 10%, semiannual coupon bond with a par value of $1,000 is currently selling for $1,365.20, producing a nominal yield to maturity of 7.5%. However, it can be called after 4 years...

Study smarter with the SolutionInn App


