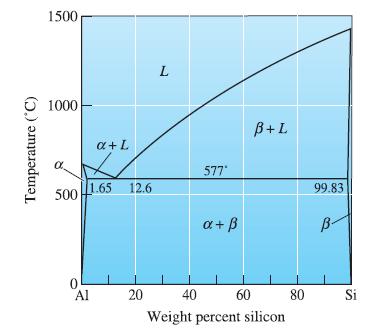
An Al-Si alloy contains 85% and 15% at 500C. Determine the composition of the alloy.
Question:
An Al-Si alloy contains 85% α and 15% β at 500°C. Determine the composition of the alloy. Is the alloy hypoeutectic or hypereutectic?
Transcribed Image Text:
Temperature (°C) 1500 1000 α 500 1.65 12.6 0 a+L Al 20 L 577" a + ß B+L 40 60 Weight percent silicon 80 99.83 B- Si
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (7 reviews)
The AlSi alloy with 85 and 15 is a hypereutectic alloy To d...View the full answer

Answered By

James Warinda
Hi! I’m James Otieno and I'm an experienced professional online tutor with countless hours of success in tutoring many subjects in different disciplines. Specifically, I have handled general management and general business as a tutor in Chegg, Help in Homework and Trans tutor accounts.
I believe that my experience has made me the perfect tutor for students of all ages, so I'm confident I can help you too with finding the solution to your problems. In addition, my approach is compatible with most educational methods and philosophies which means it will be easy for you to find a way in which we can work on things together. In addition, my long experience in the educational field has allowed me to develop a unique approach that is both productive and enjoyable.
I have tutored in course hero for quite some time and was among the top tutors awarded having high helpful rates and reviews. In addition, I have also been lucky enough to be nominated a finalist for the 2nd annual course hero award and the best tutor of the month in may 2022.
I will make sure that any student of yours will have an amazing time at learning with me, because I really care about helping people achieve their goals so if you don't have any worries or concerns whatsoever you should place your trust on me and let me help you get every single thing that you're looking for and more.
In my experience, I have observed that students tend to reach their potential in academics very easily when they are tutored by someone who is extremely dedicated to their academic career not just as a businessman but as a human being in general.
I have successfully tutored many students from different grades and from all sorts of backgrounds, so I'm confident I can help anyone find the solution to their problems and achieve
0.00
0 Reviews
10+ Question Solved
Related Book For 

The Science And Engineering Of Materials
ISBN: 9781305076761
7th Edition
Authors: Donald R. Askeland, Wendelin J. Wright
Question Posted:
Students also viewed these Engineering questions
-
The composition of moist air is given on a molar basis to be 78 percent N2, 20 percent O2, and 2 percent water vapor. Determine the mass fractions of the constituents of air.
-
The composition of moist air is given on a molar basis to be 78 percent N2, 20 percent O2, and 2 percent water vapor. Determine the mass fractions of the constituents of air.
-
The composition of timothy hay is 11.3% protein, 1.6% fat and 41% crude fiber (as-fed basis). How many pounds of protein, fat, and crude fiber are present in a ton of hay?
-
Be able to explain how land policy shaped economic growth in the United States.
-
Indicate how the following transaction is entered into the U.S. balance of payments with double-entry bookkeeping: (a) A U.S. commercial bank exchanges $800 worth of pounds sterling for dollars at...
-
A buyer received bids from three suppliers for a vital component part for its latest product. Given the following information, use total cost analysis to determine which supplier should be chosen....
-
The Carrefour Group reports the following description of its trading securities (titled financial assets reported at fair value in the income statement). Note 10 to Carrefours 2010 financial...
-
The adjusted trial balance of Business Reduction Systems at March 31, 2016, follows: Requirements 1. Journalize the required closing entries at March 31, 2016. 2. Set up T-accounts for Income...
-
Lopez Company is considering replacing one of its old manufacturing machines. The old machine has a book value of $48.000 and a remaining useful life of four years. It can be sold now for $58,000....
-
Macon Machines Company began operations on November 1, 2024. The main operating goal of the company is to sell high end robots. Customers may pay using cash or if appropriate, credit is extended to...
-
At the eutectic in the Al-Si phase diagram, what phase(s) is (are) present? Give a chemical analysis of the phase(s). Temperature (C) 1500 1000 500 1.65 0 a + L Al 12.6 20 L 577* a + B+L 40 60...
-
An Al-Si alloy contains 15% primary b and 85% eutectic microconstituent immediately after the eutectic reaction has been completed. Determine the composition of the alloy.
-
A curved piece of glass with a radius of curvature R rests on a flat plate of glass. Light of wavelength is incident normally on this system. Considering only interference between waves reflected...
-
Brice Looney owns a small retail ice cream parlor. He is considering expanding the business and has identified two attractive alternatives. One involves purchasing a machine that would enable Mr....
-
A positively charged particle initially at rest on the ground moves \(4.0 \mathrm{~m}\) upward in \(2.00 \mathrm{~s}\). If the particle has a chargeto-mass ratio of \(10 \mu \mathrm{C} / \mathrm{g}\)...
-
Central States Telecom provides communication services in Iowa, Nebraska, the Dakotas, and Montana. Central States purchased goodwill as part of the acquisition of Sheldon Wireless Company, which had...
-
Shown below is selected information from the financial records of Merris Corporation as of December 31: Required a. Determine which of the above items will appear on the statement of cash flows and...
-
Pippa runs a photographic studio specializing in black and white portrait photography. Clients book a one hour studio session and are entitled to receive two large photographs of their choice from...
-
1. Jesse wants to see a structure chart. She said to use program modules based on the processes we identified earlier. She wants the modules to be cohesive and loosely coupled. 2. Need a testing plan...
-
Arlington Merchants reported the following on its income statement for the fiscal years ending December 31, 2016 and 2015. 2016 2015 Sales $4,857,500 $4,752,900 Cost of goods sold 3,258,950 3,207,000...
-
Using activities, find the concentrations of the major species in 0.10 M NaClO 4 saturated with Mn(OH) 2 . Take the ionic strength to be 0.10 M and suppose that the ion size of MnOH + is the same as...
-
Explain why the solubility of an ionic compound increases as the ionic strength of the solution increases (at least up to ~ 0.5 M).
-
Which statements are true? In the ionic strength range 00.1 M, activity coefficients decrease with (a) Increasing ionic strength; (b) Increasing ionic charge; (c) Decreasing hydrated radius.
-
*please calculate irr in excel
-
Which of the following would not be a period cost? Research and development Direct materials Office supplies Advertising costs
-
\ table [ [ Activity Cost Pool,Activity Measure,Total Cost,Total Activity ] , [ Machining , Machine - hours,$ 3 3 0 , 0 0 0 , 1 5 , 0 0 0 MHs ] , [ Machine setups,Number of setups,$ 3 0 0 , 0 0 0 , 5...

Study smarter with the SolutionInn App


