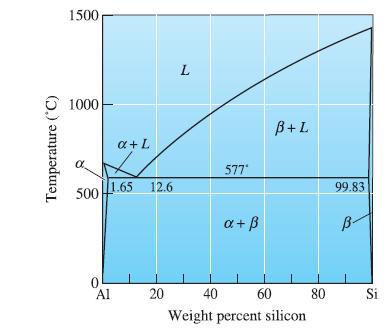
At the eutectic in the Al-Si phase diagram, what phase(s) is (are) present? Give a chemical analysis
Question:
At the eutectic in the Al-Si phase diagram, what phase(s) is (are) present? Give a chemical analysis of the phase(s).
Transcribed Image Text:
Temperature (°C) 1500 1000 500 1.65 0 a + L Al 12.6 20 L 577* a + ß B+L 40 60 Weight percent silicon 80 99.83 B- Si
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 72% (11 reviews)
ANSWER Step 1 Drawing of the aluminumsilico...View the full answer

Answered By

Aketch Cindy Sunday
I am a certified tutor with over two years of experience tutoring . I have a passion for helping students learn and grow, and I firmly believe that every student has the potential to be successful. I have a wide range of experience working with students of all ages and abilities, and I am confident that I can help students succeed in school.
I have experience working with students who have a wide range of abilities. I have also worked with gifted and talented students, and I am familiar with a variety of enrichment and acceleration strategies.
I am a patient and supportive tutor who is dedicated to helping my students reach their full potential. Thank you for your time and consideration.
0.00
0 Reviews
10+ Question Solved
Related Book For 

The Science And Engineering Of Materials
ISBN: 9781305076761
7th Edition
Authors: Donald R. Askeland, Wendelin J. Wright
Question Posted:
Students also viewed these Engineering questions
-
The copper-silver phase diagram is shown in Figure 11-30. Copper has a higher melting point than silver. Figure 11-35 A phase diagram for elements A and B (for Problem 11-36). (a) Is copper element A...
-
A PT phase diagram for potassium is shown below. a. Which phase has the higher density, the fcc or the bcc phase? Explain your answer. b. Indicate the range of P and T in the phase diagram for which...
-
What is a phase diagram? Draw a generic phase diagram and label its important features.
-
For each of these utility functions, find the optimal consumption choices z and y for a consumer with budget w = 1, who faces prices pz = 0.05 and Py = 0.3. Are the goods substitutes or complements,...
-
(a) From Table 13.3, calculate the official settlements balance of the United States for each year from 1965 to 2011. (b) Why is this appropriate measure for the U.S. balance-of-payments position...
-
Ms. Jane Kim, Purchasing Manager of Kuantan ATV, Inc., is negotiating a contract to buy 20,000 units of a common component part from a supplier. Ms. Kim has done a preliminary cost analysis on...
-
Complete the following descriptions by filling in the blanks. 1. Debt securities reflect a deposit. 2. Equity securities reflect an relationship such as investments in notes, bonds, and certificates...
-
OConnor Companys income statement information for 2016 and 2017 (a sole proprietorship) is as follows: Required: Provide the missing amounts for the blanks labeled (a) through (g). All the necessary...
-
Gary carned $57,000 as an executive. Lai lives in a nursing home. Gary received the following interest: $400 o bonds, $200 on a money market account, and $2,100 on a loan mac Gary spent one week...
-
When did the rapid development of the management science discipline begin?
-
Consider an Al-4% Si alloy. Determine (a) If the alloy is hypoeutectic or hypereutectic; (b) The composition of the first solid to form during solidification; (c) The amounts and compositions of each...
-
An Al-Si alloy contains 85% and 15% at 500C. Determine the composition of the alloy. Is the alloy hypoeutectic or hypereutectic? Temperature (C) 1500 1000 500 1.65 12.6 0 a+L Al 20 L 577" a + B+L...
-
Suppose another specimen of the soil in the preceding problem developed a major effective principal stress of 2200 kPa at failure. What would Skempton's pore pressure coefficient A at failure be, if...
-
In the circuit of Fig. 4-51 write two loop equations using I 1 and I 2 . Then find the currents and node voltages. A 3A ( 4 3 V 792 B +1 D w 392 12 C
-
The capacitor in the circuit shown in Fig. 7-37 has initial charge Q 0 = 800 C, with polarity as indicated. If the switch is closed at t = 0, obtain the current and charge, for t > 0. 100 V (+ 10 4 F
-
A gift shop sells 400 boxes of scented candles a year. The ordering cost is \($60\) for scented candles, and holding cost is \($24\) per box per year. What is the economic order size for scented...
-
Kay Vickery is angry with Gene Libby. He is behind schedule developing supporting material for tomorrows capital budget committee meeting. When she approached him about his apparent lackadaisical...
-
Tharpe Painting Company is considering whether to purchase a new spray paint machine that costs \($3,000\) . The machine is expected to save labor, increasing net income by \($450\) per year. The...
-
1. Create a detail report that will display all SCR courses in alphabetical order, with the course name and the instructor name in a group header; the Social Security number, name, and telephone...
-
Hotel Majestic is interested in estimating fixed and variable costs so that the company can make more accurate projections of costs and profit. The hotel is in a resort area that is particularly busy...
-
(a) Write the mass balance for CaCl 2 in water if the species are Ca 2+ and Cl - . (b) Write the mass balance if the species are Ca 2+ , Cl - , CaCl - , and CaOH + . (c) Write the charge balance for...
-
Write the charge and mass balances for dissolving CaF 2 in water if the reactions are CaF,(8) = Ca2+ + 2F Ca?+ + H,O = CAOH+ + H* Ca2+ + F = CaF+ CaF2(s) = CaF2(aq) F + H* = HF(aq) HF(aq) + F = HF,
-
Write charge and mass balances for aqueous Ca 3 (PO 4 ) 2 if the species are Ca 2+ , CaOH + , CaPO - 4 , PO 3- 4 , HPO 2 4 - , H 2 PO - 4 , and H 3 PO 4 .
-
1-The yield to maturity will be greater than the coupon rate when a bond is selling at a premium. Select one: a. False b. True 2-Which one of the following would have the greatest present value,...
-
! Required information [ The following information applies to the questions displayed below. ] Year 1 total cash dividends Year 2 total cash dividends Year 3 total cash dividends Year 4 total cash...
-
WISE-HOLLAND CORPORATION On June 15, 2013, Marianne Wise and Dory Holland came to your office for an initial meeting. The primary purpose of the meeting was to discuss Wise-Holland Corporation's tax...

Study smarter with the SolutionInn App


