Question: Write the charge and mass balances for dissolving CaF 2 in water if the reactions are CaF,(8) = Ca2+ + 2F Ca?+ + H,O =
Write the charge and mass balances for dissolving CaF2 in water if the reactions are
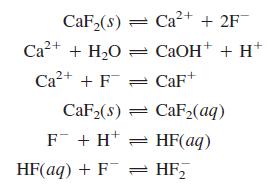
CaF,(8) = Ca2+ + 2F Ca?+ + H,O = CAOH+ + H* Ca2+ + F = CaF+ CaF2(s) = CaF2(aq) F + H* = HF(aq) HF(aq) + F = HF,
Step by Step Solution
3.54 Rating (168 Votes )
There are 3 Steps involved in it
The charge for dissolving CaF 2 in ... View full answer

Get step-by-step solutions from verified subject matter experts


