An electrochemical cell consists of a silver metal electrode immersed in a solution with [Ag + ]
Question:
An electrochemical cell consists of a silver metal electrode immersed in a solution with [Ag+] = 1.0 M separated by a porous disk from a copper metal electrode. If the copper electrode is placed in a solution of 5.0 M NH3 that is also 0.010 M in Cu(NH3)42+, what is the cell potential at 25οC?
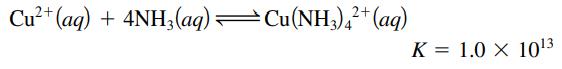
Transcribed Image Text:
Cu²+ (aq) + 4NH3(aq) —Cu(NH3)4²+ (aq) K = 1.0 X 10¹3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 72% (11 reviews)
The cell potential at 25C Ecell Ag10 M 00060 M Cu2aq50 M0010 ...View the full answer

Answered By

Brian Otieno
I'm Brian , an experienced professional freelancer with countless hours of success in freelancing many subjects in different disciplines. Specifically, I have handled many subjects and excelled in many disciplines. I have worked on many Computer Science projects and have been able to achieve a lot in that field. Additionally, I have handled other disciplines like History, Humanities, Social Sciences, Political science, Health care and life science, and Religion / Theology. My experience generally in these subjects has made me able to deliver high-quality projects in a very timely fashion. I am very reliable at my job and will get the work done in time, no matter what. In Addition, I have managed to ensure that the work meets my client's expectations and does not cause an error. I am a hard-working and diligent person who is highly responsible for everything I do. Generally, Freelancing has made me more accountable for doing my job. Additionally, I have had a passion for writing for the last seven years in this field.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
An electrochemical cell consists of a silver metal electrode immersed in a solution with [Ag + ] = 1.00 M separated by a porous disk from a compartment with a copper metal electrode immersed in a...
-
An electrochemical cell consists of a nickel metal electrode immersed in a solution with [Ni 2+ ] = 1.0 M separated by a porous disk from an aluminum metal electrode. a. What is the potential of this...
-
Gooran, Inc., has current assets of $240 million; property, plant, and equipment of $380 million; and other assets totaling $120 million. Current liabilities are $170 million and long-term...
-
How could a system be designed to allow a choice of operating systems from which to boot? What would the bootstrap program need to do?
-
Find the complex exponential Fourier series for the following signals. In each case plot the magnitude and phase line spectra for k 0. (i)x1 (i) x 1 (t) = cos(5t + 45), (ii) x 2 (t) = sin 2 (t),...
-
Describe the order in which assets must be distributed upon liquidation of a partnership, and explain the right-of-offset concept. AppendixLO1
-
The following data relate to the operations of Slick Software, Inc., during 2018. Continuing operations: Net...
-
A company's profit after tax for the year 31st December 2018 was RO 800,000. The comparative figure for the year 31st December 2017 was RO 500,000. The company's issued share capital at 1st January...
-
The following six-column table for Hawkeye Ranges includes the unadjusted trial balance as of December 31, 2013. Required 1. Complete the six-column table by entering adjustments that reflect the...
-
Cadmium sulfide is used in some semiconductor applications. Calculate the value of the solubility product constant (K sp ) for CdS given the following standard reduction potentials: CdS (s) + 2e ...
-
Consider a concentration cell that has both electrodes made of some metal M. Solution A in one compartment of the cell contains 1.0 M M 2+ . Solution B in the other cell compartment has a volume of...
-
Who can conduct cost audit under the Companies Act, 1956?
-
C 2 H 6 O 2 + NaOH + 6 H 2 O C 2 H 3 NaO 3 + O 2 + 3 H 2Hydrogen is produced at the cathode, oxYGEN AT THE ANODE .Mass balance to produce 5000 tonnes a year of glycolic acid, formic acid and oxalic...
-
Please answer: a discussion of the ethical issues involved. The court might not itself consider the ethics of the actions of the parties. However, I ask that you consider the ethics of the following:...
-
In Exercises 21-24, use these results from the "1-Panel-THC" test for marijuana use, which is provided by the company Drug Test Success: Among 143 subjects with positive test results, there are 24...
-
I need help for an assignment of a review on research on Virtual Education on study motivation and academic performance in university students. I am attaching a research article from a magazine to...
-
Shouldice Hospital in Canada is widely known for one thing-hernia repair! In fact, that is the only operation it performs, and it performs a great many of them. Over the past two decades this small...
-
Solve the equation. Give solutions in exact form. log (2 - x) = 0.5
-
Find the equations of the ellipses satisfying the given conditions. The center of each is at the origin. Passes through (2, 2) and (1, 4)
-
A bowling ball has a weight of 12 lb and the length of the lane is approximately 60. feet. Treat the ball in the lane as a one-dimensional box. What quantum number corresponds to a velocity of 7.5...
-
For a particle in a two-dimensional box, the total energy eigenfunctions are a. Obtain an expression for E nx , n y in terms of n x , n y , a, and b by substituting this wave function into the two...
-
Consider the contour plots of Problem P15.17. a. What are the most likely area or areas Îx Îy to find the particle for each of the eigenfunctions of HË depicted in plots af? b. For...
-
Callaho Inc. began operations on January 1 , 2 0 1 8 . Its adjusted trial balance at December 3 1 , 2 0 1 9 and 2 0 2 0 is shown below. Other information regarding Callaho Inc. and its activities...
-
Required: 1. Complete the following: a. Colnpute the unit product cost under absorption costing. b. What is the company's absorption costing net operating income (loss) for the quarter? c. Reconcile...
-
Bond Valuation with Semiannual Payments Renfro Rentals has issued bonds that have an 8% coupon rate, payable semiannually. The bonds mature in 6 years, have a face value of $1,000, and a yield to...

Study smarter with the SolutionInn App


