An electrochemical cell consists of a silver metal electrode immersed in a solution with [Ag + ]
Question:
An electrochemical cell consists of a silver metal electrode immersed in a solution with [Ag+] = 1.00 M separated by a porous disk from a compartment with a copper metal electrode immersed in a solution of 10.00 M NH3 that also contains 2.4 × 10-3 M Cu(NH3)42+. The equilibrium between Cu2+ and NH3 is:
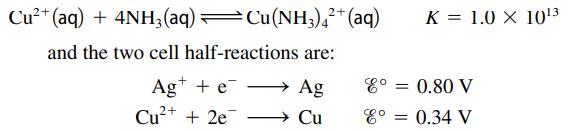
Assuming Ag+ is reduced, what is the cell potential at 25°C?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:





