Cadmium sulfide is used in some semiconductor applications. Calculate the value of the solubility product constant (K
Question:
Cadmium sulfide is used in some semiconductor applications. Calculate the value of the solubility product constant (Ksp) for CdS given the following standard reduction potentials:
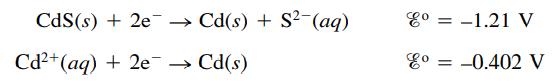
Transcribed Image Text:
CdS (s) + 2e →→ Cd(s) + S² (aq) Cd²+ (aq) + 2e → Cd(s) ४० = -1.21 V co = -0.402 V
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 70% (10 reviews)
Thorough solution with explanation The solubility product ...View the full answer

Answered By

Labindao Antoque
I graduated in 2018 with a Bachelor of Science degree in Psychology from Dalubhasaan ng Lungsod ng San Pablo. I tutored students in classes and out of classes. I use a variety of strategies to tutor students that include: lecture, discussions about the subject matter, problem solving examples using the principles of the subject matter being discussed in class , homework assignments that are directed towards reinforcing what we learn in class , and detailed practice problems help students to master a concept. I also do thorough research on Internet resources or textbooks so that I know what students need to learn in order to master what is being taught in class .
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
For the circuit in Fig. 6.70, calculate the value of R that will make the energy stored in the capacitor the same as that stored in the inductor under dc conditions. 160 uF 5A 2 4 mH
-
Calculate the value of the consumption function at each level of disposable income in Table 1 if a = 100 and mpc = 09.
-
Calculate the value of a $1,000 bond which has 10 years until maturity and pays quarterly interest at an annual coupon rate of 12 percent. The required return on similar-risk bonds is 20 percent.
-
The comparative balance sheet of Merrick Equipment Co. for December 31, 20Y9 and 20Y8, is as follows: Dec. 31, 20Y9 Dec. 31, 20Y8 Assets Cash $70,720 $47,940 Accounts receivable (net) 207,230 188,190...
-
Suppose you are a depositor at Melvin's Bank, which has the balance sheet shown in Table 10.1A. Deposit insurance does not exist. You originally deposited your money in Melvin's Bank because its...
-
The input-output equation for an analog averager is let x(t) = e j Ω0t . Since the system is LTI then the output should be y(t) = e j Ω0t H(jΩ 0 ). (a) Find the...
-
Explain the impact of a partners withdrawal from the partnership. AppendixLO1
-
Jeffrey Sammak was the owner of a contracting business known as Senaco. Sammak decided to enter the coal reprocessing business. Sammak attended the Coal Show in Chicago, Illinois, at which he met...
-
TB MC Qu. 22-106 (Algo) Ready Company has two operating... Ready Company has two operating (production) departments: Assembly and Painting. Assembly has 210 employees and occupies 56,400 square feet;...
-
On January 1, 2021, Brooks Corporation exchanged $1,255,500 fair-value consideration for all of the outstanding voting stock of Chandler, Inc. At the acquisition date, Chandler had a book value equal...
-
The electrolysis of BiO + produces pure bismuth. How long would it take to produce 10.0 g Bi by the electrolysis of a BiO + solution using a current of 25.0 A?
-
An electrochemical cell consists of a silver metal electrode immersed in a solution with [Ag + ] = 1.0 M separated by a porous disk from a copper metal electrode. If the copper electrode is placed in...
-
Use the accompanying financial statements for Fox Manufacturing Company for the year ended December 31, 2019, along with the industry average ratios below, to do the following: a. Prepare and...
-
On March 1 , Kerr Corporation issued 1 0 , 0 0 0 preferred shares for $ 1 0 0 per share. On July 1 5 , it issued an additional 3 0 , 0 0 0 shares for $ 1 2 0 per share. Each share is convertible into...
-
Hello, I need to calculate the break-even point in part B using the following formula: investment amount / (CLV of gold - CLV of platinum) . However, since that results in a negative value, does that...
-
Nervousness and depression are examples of __________ symptoms. psychophysiologic social health environmental psychological Roberta is using the structured format to present the results of his study...
-
Identify the stage of change the client is in and write in behavioral language at least one problem, with at least one goal and a minimum of two objectives for each goal for the client vignettes...
-
Photon Technologies, Inc., a manufacturer of batteries for mobile phones, signed a contract with a large electronics manufacturer to produce three models of lithium-ion battery packs for a new line...
-
Solve the equation. Give solutions in exact form. log (3 - x) = 0.75
-
Determine the center and radius of each circle. Sketch each circle. 4x 2 + 4y 2 9 = 16y
-
Reaction of iodoethane with CN yields a small amount of isonitrile, CH3CH2N C, along with the nitrile CH3CH2C N as the major product. Write electron-dot structures for both products, assign formal...
-
A Alkynes can be made by dehydrohalogenation of vinylic halides in a reaction that is essentially an E2 process. In studying the stereochemistry of this elimination, it was found that...
-
(S)-2-Butanol slowly racemizes on standing in dilute sulfuric acid.Explain. CH3CH2CHCH3 2-Butanol
-
Cash from Operating Activities: ______________ Cash from Investing Activities: ______________ Cash from Financing Activities: ______________ Problem 2: Financial Ratios The GAP Macys 1 Current Ratio...
-
On January 1, 2021, Winky Enterprises issued 12% bonds dated January 1, 2021, with a face amount of $2,800,000. The bonds mature in 2030 (10 years). For bonds of similar risk and maturity, the market...
-
Using the following accounts and balances, prepare the stockholders' equity vection of the balance sheet. Pilty thousand shares of common stock are authorised, and 1,000 shares have been recoured,...

Study smarter with the SolutionInn App


