An organometallic compound is one containing at least one metalcarbon bond. An example of an organometallic species
Question:
An organometallic compound is one containing at least one metal–carbon bond. An example of an organometallic species is (CH3CH2)MBr, which contains a metal–ethyl bond.
a. If M2+ has the electron configuration [Ar]3d10, what is the percent by mass of M in (CH3CH2)MBr?
b. A reaction involving (CH3CH2)MBr is the conversion of a ketone to an alcohol as illustrated here:
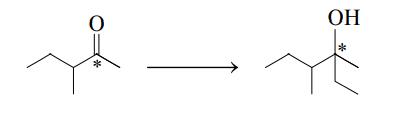
How does the hybridization of the starred carbon atom change, if at all, in going from reactants to products?
c. What is the systematic name of the product?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:





