Esterification reactions are carried out in the presence of a strong acid such as H 2 SO
Question:
Esterification reactions are carried out in the presence of a strong acid such as H2SO4. A carboxylic acid is warmed with an alcohol, and an ester and water are formed. You may have made a fruity-smelling ester in the lab when studying organic functional groups. Name the carboxylic acid that is necessary to complete the following esterification reaction.
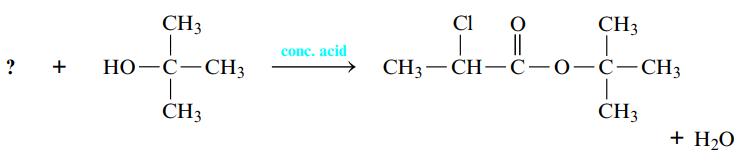
Transcribed Image Text:
? + CH3 | HO-C-CH3 T CH3 conc. acid Cl O 98 || CH3—CH—C—0—C—CH3 CH3 1 I CH3 + H₂O
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (12 reviews)
The carboxylic acid that is necessary is Acetic Acid Esterifi...View the full answer

Answered By

Brian Otieno
I'm Brian , an experienced professional freelancer with countless hours of success in freelancing many subjects in different disciplines. Specifically, I have handled many subjects and excelled in many disciplines. I have worked on many Computer Science projects and have been able to achieve a lot in that field. Additionally, I have handled other disciplines like History, Humanities, Social Sciences, Political science, Health care and life science, and Religion / Theology. My experience generally in these subjects has made me able to deliver high-quality projects in a very timely fashion. I am very reliable at my job and will get the work done in time, no matter what. In Addition, I have managed to ensure that the work meets my client's expectations and does not cause an error. I am a hard-working and diligent person who is highly responsible for everything I do. Generally, Freelancing has made me more accountable for doing my job. Additionally, I have had a passion for writing for the last seven years in this field.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
In the presence of a platinum catalyst, ammonia, NH3, burns in oxygen, O2, to give nitric oxide, NO, and water vapor. How many volumes of nitric oxide are obtained from one volume of ammonia,...
-
When ethyl 4-hydroxybutyrate is heated in the presence of a trace of a basic catalyst (sodium acetate), one of the products is a lactone. Propose a mechanism for formation of this lactone.
-
In the presence of a tungsten catalyst at high temperatures, the decomposition of ammonia to nitrogen and hydrogen is a zero-order process. If the rate constant at a particular temperature is 3.7 ...
-
20 -101 10 in- laminate substrate Fig.2 Q2: The tool shown in Fig.2 is used in a gluing operation to press a thin laminate to a thicker substrate. If the wheels at points A and B both have 2 in...
-
The Triton Energy Corporation explores for and produces oil and gas. Company president Gail Freeman wants to have her company's analyst forecast the company's sales per share for 2000. This will be...
-
In problem, use the graph shown to find (a) The domain and range of each function (b) The intercepts, if any (c) Horizontal asymptotes, if any (d) Vertical asymptotes, if any (e) Oblique asymptotes,...
-
What is a bond indenture? What provisions are usually included in it? AppendixLO1
-
On January 1, 2014, Prince Corporation acquired 70% of the 100,000 outstanding voting shares of Song Limited for a cash consideration of $1,015,000. On that date, shares of Song Limited were trading...
-
In your opinion, what is the primary job of the HR professional in an organization & why is HR important. Please list 3-4 items that you feel HR professionals are involved in. Also, explain why an...
-
Angela Care Homes Ltd. (Angela) operates 14 care homes for people suffering with mental illness in Western Canada. It hopes to expand to Eastern Canada over the next few years and is considering...
-
An organometallic compound is one containing at least one metalcarbon bond. An example of an organometallic species is (CH 3 CH 2 )MBr, which contains a metalethyl bond. a. If M 2+ has the electron...
-
Ignoring ring compounds, which isomer of C 2 H 4 O 2 should boil at the lowest temperature?
-
In each of the following independent situations, an example is given requiring a trade-off between the qualitative characteristics discussed in the text. For each situation, identify the relevant...
-
1. Consider the following economy: C = 3, I = 1.5, G = 2.65, T = 2, f = 0.5, d = 0.1, a = 0.8 a) Write the mathematical expression of the consumption function b) Write the mathematical expression of...
-
Question 2 (Financial statement Analysis) Following is a comparative statement of financial position for Sam's Company: Sam's Company Comparative Statement of Financial Position December 31, 2020 and...
-
Q4. Johnny's Burger is a family-run fast food joint. In addition to its famous hamburger, Johnny's Burger has just launched a new "Organic Beef burger. The owner, Johnny, would like to know if his...
-
Compute ScholarPak's break-even point in sales dollars for the year. 2. Compute the number of sales units required to earn a net income of $540,000 during the year. 3. ScholarPak's variable...
-
41-44 Find fogoh. 41. f(x)=3x-2, g(x) = sin x, 42. f(x)=|x4|, g(x) = 2, 43. f(x)=x-3, g(x) = x, h(x) = x h(x) = x h(x) = x + 2 44. f(x) = tan x, g(x) == X x-1' h(x) = x
-
During World War II, the insecticide DDT was used successfully to halt a typhus epidemic spread by lice and to control mosquitoes and flies. After World War II, it was used extensively to control...
-
The following cost information was provided to you for analysis: September 12,000 Units Produced Costs: TIC TAC TOE TING August 10,000 P80,000 70.000 60.000 50,000 How much is the fixed cost per...
-
Write the structure of a representative segment of polyurethane prepared by reaction of ethylene glycol with MDI.
-
The smoking salons of the Hindenburg and other hydrogen-filled dirigibles of the 1930s were insulated with urea formaldehyde polymer foams. The structure of this polymer is highly cross-linked, like...
-
The polymeric resin used for Merrifield solid-phase peptide synthesis (Section 26.8) is prepared by treating polystyrene with N-(hydroxymethyl) phthalimide and trifluoromethanesulfonic acid, followed...
-
Based on the regression output (below), would you purchase this actively managed fund with a fee of 45bps ? Answer yes or no and one sentence to explain why.
-
What is the yield to maturity on a 10-year, 9% annual coupon, $1,000 par value bond that sells for $967.00? That sells for $1,206.10?
-
1)Prepare the journal entry to record Tamas Companys issuance of 6,500 shares of $100 par value, 9% cumulative preferred stock for $105 cash per share. 2. Assuming the facts in part 1, if Tamas...

Study smarter with the SolutionInn App


