Balance each of the following chemical equations. a. KO (s) + HO(1)KOH(aq) + O(g) + HO(aq) b.
Question:
Balance each of the following chemical equations.
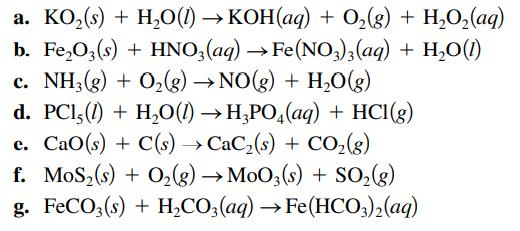
Transcribed Image Text:
a. KO₂ (s) + H₂O(1)→KOH(aq) + O₂(g) + H₂O₂(aq) b. Fe₂O3(s) + HNO3(aq) → Fe(NO3)3(aq) + H₂O(1) c. NH3(g) + O₂(g) → NO(g) + H₂O(g) d. PC1,(1) + H₂O(1)→ H3PO4(aq) + HCl(g) e. CaO(s) + C(s) →CaCz(s) + CO,(g) f. MoS₂ (s) + O₂(g) → MoO3(s) + SO₂(g) g. FeCO3(s) + H₂CO3(aq) →Fe(HCO3)2(aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 35% (14 reviews)
2KO2s 2H2Ol 4KOHaq O2g 2H2O2aq Fe2O3s 6...View the full answer

Answered By

Tobias sifuna
I am an individual who possesses a unique set of skills and qualities that make me well-suited for content and academic writing. I have a strong writing ability, allowing me to communicate ideas and arguments in a clear, concise, and effective manner. My writing is backed by extensive research skills, enabling me to gather information from credible sources to support my arguments. I also have critical thinking skills, which allow me to analyze information, draw informed conclusions, and present my arguments in a logical and convincing manner. Additionally, I have an eye for detail and the ability to carefully proofread my work, ensuring that it is free of errors and that all sources are properly cited. Time management skills are another key strength that allow me to meet deadlines and prioritize tasks effectively. Communication skills, including the ability to collaborate with others, including editors, peer reviewers, and subject matter experts, are also important qualities that I have. I am also adaptable, capable of writing on a variety of topics and adjusting my writing style and tone to meet the needs of different audiences and projects. Lastly, I am driven by a passion for writing, which continually drives me to improve my skills and produce high-quality work.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
Balance each of the following chemical equations. a. KO2(s) + H2O(l) ( KOH(aq) + O2(g) + H2O2(aq) b. Fe2O3(s) + HNO3(aq) ( Fe(NO3)3(aq) + H2O(l) c. NH3(g) + O2(g) ( NO(g) + H2O(g) d. PCl5(l) + H2O(l)...
-
Balance each of the following oxidation reduction reactions by using the oxidation states method. a. C2H6(g) + O2(g) CO2(g) + H2O(g) b. Mg(s) + HCl(aq) Mg2+(aq) + Cl2(aq) + H2(g) c. Cu(s) + Ag+(aq)...
-
Each of the following equations describes a reaction of a compound called methyl formate. To what class of compounds does methyl formate belong? Which reactions require a reducing agent? Which...
-
IfAUB=AUC and An B=An C, then B = C. Statement-2 AU (BOC) = (AUB) n (AUC)
-
Beth retires when she turns 65. She begins receiving a monthly pension of $300 from her employers qualified retirement plan. While employed, Beth contributed $13,000 to the plan. a. Beth uses the...
-
How would you differentiate between corporate responsibility and corporate sustainability?
-
E3-4 Correction of consolidated net income Liong Corporation paid $2,500,000 in cash for an 80 percent interest in Taro Corporation on January 1, 2016, when the book value of Taros net assets was...
-
Nuke-It-Now manufactures microwave ovens. The following represents the financial information from one of its manufacturing plants for two years. Required a. Classify these items into prevention (P),...
-
Note: This problem is for the 2020 tax year. Alfred E. Old and Beulah A. Crane, each age 42, married on September 7, 2018. Alfred and Beulah will file a joint return for 2020. Alfred's Social...
-
The B. Hall Real Estate Investment Corporation has identified four small apartment buildings in which it would like to invest. Mrs. Hall has approached three savings and loan companies regarding...
-
Balance the following equations representing combustion reactions: a. b. (0) + (g) OH (g) + C 90 (g) (g) + (g) + c. C2H2O11(s) + O(g) CO(g) + HO(g) d. Fe(s) + O(g) FeO3(s) e. FeO(s) + O(g) FeO3(s)...
-
Iron oxide ores, commonly a mixture of FeO and Fe 2 O 3 , are given the general formula Fe 3 O 4 . They yield elemental iron when heated to a very high temperature with either carbon monoxide or...
-
In practice, what is the most serious problem raised by real options?
-
Locate a scholarly article relevant to how to present your financial plan for opening a Roller Skating Rink (from your draft business plan) to a lending institution--and describe your strategy for...
-
How would you expect seasonal fluctuations in demand to affect a rental company's decisions about pricing rented products such as wedding dresses or convertible cars? In terms of pricing principles,...
-
Do we drive technology, or does technology drive us? If technology drives us, what are the risks? The other side of the coin would be that we are able to stay ahead of technological transformations....
-
How do you explain the differences between the two analyses and what are the implications of using the BCG matrix in practice?
-
How do leadership styles, such as transformational leadership, shared leadership, and servant leadership, impact team dynamics, member motivation, and overall team effectiveness ?
-
1. Could annoyance to the public ever rebound as a direct cost to the firm? 2. Choose two products which are extensively advertised. Make out a case for and a case against these particular...
-
An auto-parts manufacturer is considering establishing an engineering computing center. This center will be equipped with three engineering workstations each of which would cost $25,000 and have a...
-
Calculate the thermal conductivity of nitrogen (C; m = 20.8 J K-1 mol-1, a= 0.43 nm3) at room temperature (20C).
-
Calculate the diffusion constant of nitrogen at 25C and (a) 10,0 Pa, (b) 100 kPa, (c) 15.0 MPa. If a pressure gradient 01'0.20 bar m-1 is established in a pipe, what is the flow of gas due to...
-
The mobility of an acetate ion in aqueous solution at 25C is 4.24 x 10-8 m3 S-1 V-1. Calculate the molar ionic conductivity.
-
THIS IS ONE QUESTION WITH TWO PARTS. PLEASE ANSWER COMPLETELY AND SHOW ALL WORK. (NO EXCEL) Information for Question 1: State Probability Retum on A Return on B Return on C Retum on Portfolio X Boom...
-
Direct materials (5.0 Ibs. @ $5.00 per Ib.) Direct labor (2.0 hrs. @ $13.00 per hr.) Overhead (2.0 hrs. @ $18.50 per hr.) Total standard cost $25.00 26.00 37.00 $88.00 The predetermined overhead rate...
-
Problem 1-28 (Algo) (LO 1-4, 1-5, 1-6b 1-7) Harper, Inc., acquires 40 percent of the outstanding voting stock of Kinman Company on January 1, 2020, for $316,100 in cash. The book value of Kinman's...

Study smarter with the SolutionInn App


