Iron oxide ores, commonly a mixture of FeO and Fe 2 O 3 , are given the
Question:
Iron oxide ores, commonly a mixture of FeO and Fe2O3, are given the general formula Fe3O4. They yield elemental iron when heated to a very high temperature with either carbon monoxide or elemental hydrogen. Balance the following equations for these processes:
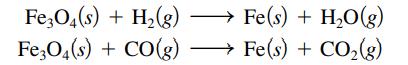
Transcribed Image Text:
Fe3O4(s) + H₂(g) Fe3O4(s) + CO(g) Fe(s) + H₂O(g) Fe(s) + CO₂(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (14 reviews)
Fe3O4s 4H2g Fes 4H2Og Fe3O4s 3COg Fes 3CO2g The above equations are balanced ...View the full answer

Answered By

Labindao Antoque
I graduated in 2018 with a Bachelor of Science degree in Psychology from Dalubhasaan ng Lungsod ng San Pablo. I tutored students in classes and out of classes. I use a variety of strategies to tutor students that include: lecture, discussions about the subject matter, problem solving examples using the principles of the subject matter being discussed in class , homework assignments that are directed towards reinforcing what we learn in class , and detailed practice problems help students to master a concept. I also do thorough research on Internet resources or textbooks so that I know what students need to learn in order to master what is being taught in class .
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
As a body is heated to a very high temperature and becomes self luminous, the apparent color of the emitted radiation shifts from red to yellow and finally to blue as the temperature increases. Why...
-
Moist air is heated to a very high temperature. If the equilibrium composition consists of H2O, O2, N2, OH, H2, and NO, the number of equilibrium constant relations needed to determine the...
-
A mixture of Fe2O3 and FeO was found to contain 72.00% Fe by mass. What is the mass of Fe2O3 in 0.500 g of this mixture?
-
Give a recursive algorithm for finding the reversal of a bit string. (See the definition of the reversal of a bit string in the preamble of Exercise 34 in Section 5.3.)
-
Tim retired during the current year at age 58. He purchased an annuity from American National Life Company for $40,000. The annuity pays Tim $500 per month for life. a. Compute Tims annual exclusion....
-
Should we worry about the fact that the United States is a debtor nation?
-
3. In Pops December 31, 2016, consolidated balance sheet, what amount should be reported as total retained earnings? a $2,480,000 b $2,720,000 c $2,760,000 d $3,600,000
-
Satu Company, a merchandiser, recently completed its 2015 operations. For the year, (1) all sales are credit sales, (2) all credits to Accounts Receivable reflect cash receipts from customers, (3)...
-
A stock is bought for $30 and sold for $40 one year later, immediately after it has paid a dividend of $1.50. The capital gain rate for this transaction is ____%.
-
Hotel Baroneii International is an international hospitality group that is present in more than 100 countries with 12 brands and 5000 plus properties. The group also has a strong loyalty membership...
-
Balance each of the following chemical equations. a. KO (s) + HO(1)KOH(aq) + O(g) + HO(aq) b. FeO3(s) + HNO3(aq) Fe(NO3)3(aq) + HO(1) c. NH3(g) + O(g) NO(g) + HO(g) d. PC1,(1) + HO(1) H3PO4(aq) +...
-
A common demonstration in chemistry courses involves adding a tiny speck of manganese(IV) oxide to a concentrated hydrogen peroxide (H 2 O 2 ) solution. Hydrogen peroxide decomposes quite...
-
For each of the following situations, state whether the mean would be a statistic or a parameter. Explain your answer. a. According to Canadian census data, the median family income in British...
-
How do these relevant legal principles apply: Duty of care Duty of obedience Duty of loyalty Shareholder Derivative suit Piercing the corporate veil...
-
what will you do as a hotel manager if a customer complained about bad service they received?
-
How do marketers use new products to maintain and grow their market share? Your response must include a specific example of a company that successfully grew its business or attracted a new target...
-
How do you encourage cross-functional synergy within your organization to break down silos and facilitate innovative solutions to complex challenges ?
-
1. what is intended internal resource strategies. How do you plan to develop or acquire resources (tangible and/or intangible) that would generate core competencies? What are examples of resource...
-
1. In what stages of their life cycle are the following products: Typewriters; Nintendo DS; digital watches; coal; clockwork watches; jeans; small tall saloon cars; smart TVs; electric bicycles;...
-
Design an experiment to demonstrate that RNA transcripts are synthesized in the nucleus of eukaryotes and are subsequently transported to the cytoplasm.
-
The mobility of a u- ion in aqueous solution is 4.01 x 10-8 m2 S-1 V-1 at 25C. The potential difference between two electrodes placed in the solution is 12.0 V. If the electrodes are 1.00 cm apart,...
-
What fraction of the total current is carried by er when current flows through an aqueous solution of NaCI at 25C?
-
What fraction of the total current is carried by er when current flows through an aqueous solution of NaCI at 25C?
-
Question 3 (24 marks) Wonderful Technology Company Limited sells computers and accessories. Data of the store's operations are as follow: Sales are budgeted at $400,000 for December 2019, $420,000...
-
Kratz Manufacturing Company uses an activity-based costing system. It has the following manufacturing activity areas, related cost drivers and cost allocation rates: Activity Cost Driver Cost...
-
You are a Partner with Fix-It Consultants and have been engaged in an advisory capacity with a software company, called MoveFast. The company is seeing a sharp decline in revenue, with the primary...

Study smarter with the SolutionInn App


