Balance the following equations representing combustion reactions: a. b. (0) + (g) OH (g) + C 90
Question:
Balance the following equations representing combustion reactions:
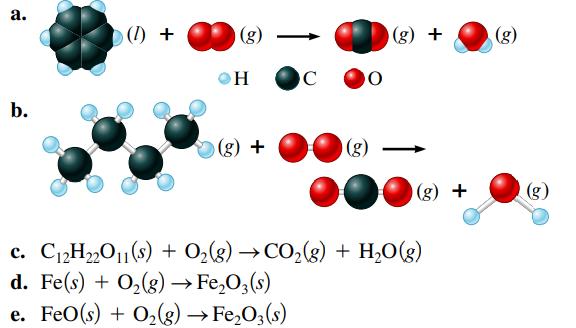
Transcribed Image Text:
a. b. (0) + (g) OH (g) + C 90 (g) (g) + (g) + c. C₁2H₂2O11(s) + O₂(g) →CO₂(g) + H₂O(g) d. Fe(s) + O₂(g) → Fe₂O3(s) e. FeO(s) + O₂(g) → Fe₂O3(s) (g) (g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 72% (11 reviews)
ANSWER Balancing a chemical equation involves adjusting the coefficients numbers in front of each mo...View the full answer

Answered By

Dennis Nyangau
I have been tutoring for several years now, and I absolutely love it! I love being able to help students one-on-one and see them succeed. It is so gratifying to see a student understand a concept that they were struggling with before. I also enjoy getting to know my students and helping them to reach their full potential.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
Balance the following equations and indicate whether they are combination, decomposition, or combustion reactions: (a) C3H6(g) + O2 (g) CO2 (g) + H2O(g) (b) NH4NO3(s) N2O(g) + H2O(g) (c) C5H6O(I) +...
-
Balance the following equations and indicate whether they are combination, decomposition, or combustion reactions: (a) PbCO3(s) PbO(s) + CO2(g) (b) C2H4(g) + O2(g) CO2(g) + H2O(g) (c) Mg(s) + N2(g)...
-
Balance the following equations and write the corresponding ionic and net ionic equations (if appropriate): (a) (b) (c) Ba(OH)-(aq) + HPO 4 (aq )- HCIO4 (aq) + Mg(OH )2 (s)
-
Show that 15 is an inverse of 7 modulo 26.
-
Dan and Diana file a joint return. Dan earned $31,000 during the year before losing his job. Diana received Social Security benefits of $5,000. a. Determine the taxable portion of the Social Security...
-
Provide examples of how the self-reference criterion might manifest itself.
-
E3-5 Consolidated Balance Sheet after Acquisition On January 1, 2014, Wins Inc. acquired a 70 percent interest in Matt Inc. at a cost of $1,400,000. Matt Inc.s net assets on this date were...
-
How would each of the following changes tend to affect aggregate payout ratios (that is, the average for all corporations), other things held constant? Explain your answers. a. An increase in the...
-
Puget Sound Divers is a company that provides diving services such as underwater ship repairs to clients in the Puget Sound area. The companys planning budget for May appears below: During May, the...
-
Consider a welfare program (such as SNAP) with benefits that decrease as an individuals income increases. Draw the individuals budget constraint with and without the subsidy. (Put hours of work on...
-
Glass is a mixture of several compounds, but a major constituent of most glass is calcium silicate, CaSiO 3 . Glass can be etched by treatment with hydrofluoric acid; HF attacks the calcium silicate...
-
Balance each of the following chemical equations. a. KO (s) + HO(1)KOH(aq) + O(g) + HO(aq) b. FeO3(s) + HNO3(aq) Fe(NO3)3(aq) + HO(1) c. NH3(g) + O(g) NO(g) + HO(g) d. PC1,(1) + HO(1) H3PO4(aq) +...
-
What is the relationship between covariance and the correlation coefficient?
-
Write down at leastfive items (durable goods, not food) that you purchase and their sourcing (where each is from). For example, a shirt may be assembled in China, designed in the US, and made from...
-
I agree that overtime can be tricky in different countries. As we've seen with piecework, it is hard to implement it in countries where people are not motivated to work past their regular hours, even...
-
time (in seconds). Find a formula for 1, if V = 5t(t Suppose that an object's acceleration function is given by a = 4t+ 6. The object's initial velocity is 4, and the initi position is 9. Find the...
-
Determine the specific major and foundational managerial discoveries and findings from each era as most pivotal for management evolution (Early Management Era, Social Management Era, Scientific...
-
Identify the three major pricing strategies and discuss the important key factors that impact setting prices. Explain what time of pricing strategy your assigned brand uses and why you believe this...
-
Assume that a firm faces a downward-sloping demand curve. Draw a diagram showing the firms AR, MR, AC and MC curves. (Draw them in such a way that the firm can make supernormal profits.) Mark the...
-
A red card is illuminated by red light. What color will the card appear? What if its illuminated by blue light?
-
Calculate the viscosity of benzene vapour at (a) 273 K, (b) 298 K, (c) 1000 K. Take a~ 0.88 nM-1.
-
Calculate the thermal conductivities of (a) Neon, (b) Nitrogen at 300 K and 15 mbar. Each gas is confined in a cubic vessel of side 15 cm, one wall being at 305 K and the one opposite at 295 K. What...
-
The viscosity of a chlorofluorocarbon (CFC) was measured by comparing its rate of flow through a long narrow tube (using Poiseuille formula) with that of argon. For the same pressure differential,...
-
Just work out the assignment on your own sheet, you dont need the excel worksheet. Classic Coffee Company Best friends, Nathan and Cody, decided to start their own business which would bring great...
-
Financial information related to the proprietorship of Ebony Interiors for February and March 2019 is as follows: February 29, 2019 March 31, 2019 Accounts payable $310,000 $400,000 Accounts...
-
(b) The directors of Maureen Company are considering two mutually exclusive investment projects. Both projects concern the purchase of a new plant. The following data are available for each project...

Study smarter with the SolutionInn App


