Balance the following oxidationreduction reactions that occur in basic solution. a. Cr(s) + CrO2 (aq) Cr(OH)3(s)
Question:
Balance the following oxidation–reduction reactions that occur in basic solution.
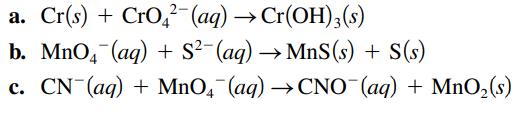
Transcribed Image Text:
a. Cr(s) + CrO2 (aq) → Cr(OH)3(s) b. MnO4 (aq) + S²(aq) →MnS (s) + S(s) c. CN (aq) + MnO4 (aq) →CNO (aq) + MnO₂ (s)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 58% (12 reviews)
a Crs CrO4aq 8OHaq CrOH3s 4H2Ol b MnO4 aq S2 aq 2OHaq MnS s Ss H2Ol c CN aq MnO4 aq ...View the full answer

Answered By

Labindao Antoque
I graduated in 2018 with a Bachelor of Science degree in Psychology from Dalubhasaan ng Lungsod ng San Pablo. I tutored students in classes and out of classes. I use a variety of strategies to tutor students that include: lecture, discussions about the subject matter, problem solving examples using the principles of the subject matter being discussed in class , homework assignments that are directed towards reinforcing what we learn in class , and detailed practice problems help students to master a concept. I also do thorough research on Internet resources or textbooks so that I know what students need to learn in order to master what is being taught in class .
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
Balance each of the following oxidation reduction reactions by using the oxidation states method. a. C2H6(g) + O2(g) CO2(g) + H2O(g) b. Mg(s) + HCl(aq) Mg2+(aq) + Cl2(aq) + H2(g) c. Cu(s) + Ag+(aq)...
-
Balance these redox reactions that occur in aqueous solution. Use whatever water-derived species is necessary; there may be more than one correct balanced equation. a. CrO3 + Ni2+ ( Cr3+ + Ni3+ b....
-
Balance these redox reactions that occur in aqueous solution. Use whatever water-derived species is necessary; there may be more than one correct balanced equation. a. ClO + Ti2+ ( Ti4+ + Cl b. BrO3...
-
You have the following information on two bonds Bond 1 Bond 1 Type Coupon Zero Coupon Term (Yrs) 3 3 Payment Frequency Annual Annual Face Amount ($) $25,000 $25,000 Coupon (%) 6% NA a. Calculate the...
-
In 2002, Florence purchased 30 acres of land. She has not used the land for business purposes or made any substantial improvements to the property. During the current year, she subdivides the land...
-
Based on the Michigan Income Dynamics Study, Hausman attempted to estimate a wage, or earnings, model using a sample of 629 high school graduates, who were followed for a period of six years, thus...
-
Calculate Material variances from the following data: Material Standard Actual Std. Qty. Rate Amount Qty. Rate Amount A 10 2 20 5 3 15 B 20 3 60 10 6 60 C 20 6 120 15 5 75 50 200 30 150 [Ans: MCV =...
-
Use the file statepop.dat for this problem. a. Plot the total number of veterans versus the probabilities of selection i. Does your plot indicate that unequal-probability sampling will be helpful...
-
Jefferson Memorial Hospital is an investment center as a division of Hospitals United. During the past year, Jefferson reported an after-tax income of $7.2 million. Total interest expense was...
-
Lisbon Company SA lost 70% of its inventory in a fire on March 25, 2017. The accounting records showed the following gross profit data for February and March. Lisbon Company is fully insured for fire...
-
Chlorine gas was first prepared in 1774 by C. W. Scheele by oxidizing sodium chloride with manganese(IV) oxide. The reaction is Balance this equation. NaCl(aq) + HSO4 (aq) + MnO (s) NaSO4 (aq) +...
-
Specify which of the following equations represent oxidation reduction reactions, and indicate the oxidizing agent, the reducing agent, the species being oxidized, and the species being reduced. a....
-
A market has an inverse demand curve p = 100 2Q and four firms, each of which has a constant marginal cost of MC = 20. If the firms form a profit-maximizing cartel and agree to operate subject to...
-
1. Consider the following economy: C = 3, I = 1.5, G = 2.65, T = 2, f = 0.5, d = 0.1, a = 0.8 a) Write the mathematical expression of the consumption function b) Write the mathematical expression of...
-
Question 2 (Financial statement Analysis) Following is a comparative statement of financial position for Sam's Company: Sam's Company Comparative Statement of Financial Position December 31, 2020 and...
-
Q4. Johnny's Burger is a family-run fast food joint. In addition to its famous hamburger, Johnny's Burger has just launched a new "Organic Beef burger. The owner, Johnny, would like to know if his...
-
Compute ScholarPak's break-even point in sales dollars for the year. 2. Compute the number of sales units required to earn a net income of $540,000 during the year. 3. ScholarPak's variable...
-
41-44 Find fogoh. 41. f(x)=3x-2, g(x) = sin x, 42. f(x)=|x4|, g(x) = 2, 43. f(x)=x-3, g(x) = x, h(x) = x h(x) = x h(x) = x + 2 44. f(x) = tan x, g(x) == X x-1' h(x) = x
-
1. Dan Ariely suggested that bonuses should be based on an average of the previous five years performance. Explain why this might lead to better performance than a simple annual bonus. 2. In Chapter...
-
When you weigh yourself on good old terra firma (solid ground), your weight is 142 lb. In an elevator your apparent weight is 121 lb. What are the direction and magnitude of the elevator's...
-
Calculate the magnetic field needed to satisfy the resonance condition for unshielded protons in a 150.0 MHz radiofrequency field.
-
Use Table 15.2 to predict the magnetic fields at which (a) 14N, (b) 19F, and (c) 31p comes into resonance at (i) 300 MHz, (ii) 750 MHz.
-
Calculate the relative population differences (oN/N) for 13Cnuclei in fields of (a) 0.50 T, (b) 2.5 T, and (c) 15.5 T at 25C.
-
ABC Company engaged in the following transaction in October 2 0 1 7 Oct 7 Sold Merchandise on credit to L Barrett $ 6 0 0 0 8 Purchased merchandise on credit from Bennett Company $ 1 2 , 0 0 0 . 9...
-
Lime Corporation, with E & P of $500,000, distributes land (worth $300,000, adjusted basis of $350,000) to Harry, its sole shareholder. The land is subject to a liability of $120,000, which Harry...
-
A comic store began operations in 2018 and, although it is incorporated as a limited liability company, it decided to be taxed as a corporation. In its first year, the comic store broke even. In...

Study smarter with the SolutionInn App


