Concentrated hydrogen peroxide solutions are explosively decomposed by traces of transition metal ions (such as Mn or
Question:
Concentrated hydrogen peroxide solutions are explosively decomposed by traces of transition metal ions (such as Mn or Fe):
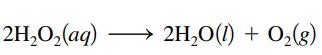
What volume of pure O2(g), collected at 27οC and 746 torr, would be generated by decomposition of 125 g of a 50.0% by mass hydrogen peroxide solution? Ignore any water vapor that may be present.
Transcribed Image Text:
2H₂O₂(aq) 2H₂O(1) + O₂(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 45% (11 reviews)
Volume 050 Jg ...View the full answer

Answered By

Brian Otieno
I'm Brian , an experienced professional freelancer with countless hours of success in freelancing many subjects in different disciplines. Specifically, I have handled many subjects and excelled in many disciplines. I have worked on many Computer Science projects and have been able to achieve a lot in that field. Additionally, I have handled other disciplines like History, Humanities, Social Sciences, Political science, Health care and life science, and Religion / Theology. My experience generally in these subjects has made me able to deliver high-quality projects in a very timely fashion. I am very reliable at my job and will get the work done in time, no matter what. In Addition, I have managed to ensure that the work meets my client's expectations and does not cause an error. I am a hard-working and diligent person who is highly responsible for everything I do. Generally, Freelancing has made me more accountable for doing my job. Additionally, I have had a passion for writing for the last seven years in this field.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
Explain why hydrated metal ions such as (H 2 O) 6 Fe 3 + hydrolyze to give H + , but hydrated anions such as (H 2 O) 6 Cl= do not hydrolyze to give H + .
-
Gas at 27 C and 5 MPa expands adiabatically against a pressure of 1 atmosphere in such a way that the final volume triples. What is the final temperature of the system? Calculate Q, W, E, H. Cp = 5/2...
-
Hydrogen peroxide undergoes a first-order decomposition to water and O2 in aqueous solution. The rate constant at 25oC is 7.40 104/s. Calculate the volume of O2 obtained from the decomposition...
-
Cooper Movie Studio Corp. makes movies and is interested in lowering its operating costs for the following year, while maintaining the high quality and appeal of its movies. Cooper's management is...
-
Mollie Morrow was a real estate agent working for Commercial Properties. She was approached by Shane Jacobs to find a commercial building in downtown Halifax. She did some investigating and found a...
-
On average, do people gain weight as they age? Using data from the same study as in Exercise 11-1, we provide some summary statistics for both age and weight. (a) Calculate the least squares...
-
7 Outline the stages through which organisations go in using the internet, giving an original example of each.
-
Squeezers Juice and Tea Company manufactures juices and chai teas that are sold at organic foods stores. Several of its products have been featured in movies because the companys products are popular...
-
E9-9 (Algo) Calculating Direct Labor Variances [LO 9-4) Parker Plastic, Inc., manufactures plastic mats to use with rolling office chairs. Its standard cost information for last year follows Direct...
-
It is late 2019, and you are a successful executive working in New York for a large company. Tomorrow morning you will have the opportunity to negotiate receiving a $100,000 bonus at the end of the...
-
A 2.50-L container is filled with 175 g argon. a. If the pressure is 10.0 atm, what is the temperature? b. If the temperature is 225 K, what is the pressure?
-
Consider two separate gas containers at the following conditions: How is the pressure in container B related to the pressure in container A? Container A Contents: SO(g) Pressure = PA Container B...
-
Use mesh analysis to find currents I1, I2, and I 3 in the circuit of Fig. 10.82. 120/90 V ( 120 230 V
-
Which alternative strategy do each of the following fall under? 1. Nike could set more aggressive sustainability targets and timelines for each product category, allocating additional resources to...
-
Find the critical value Za/2 that corresponds to the given confidence level. 88%
-
A study was conducted to determine the proportion of people who dream in black and white instead of color. Among 296 people over the age of 55, 73 dream in black and white, and among 294 people under...
-
The other strategy could be to develop a completely distinct product line. This would allow Nike to develop sustainable products without affecting their main products. It could target specific green...
-
A drug is used to help prevent blood clots in certain patients. In clinical trials, among 4705 patients treated with the drug, 170 developed the adverse reaction of nausea. Construct a 95% confidence...
-
A dietitian at Palos Community Hospital wants a patient to have a meal that has 78 grams (g) of protein, 59 g of carbohydrates, and 75 milligrams (mg) of vitamin A. The hospital food service tells...
-
Define relevant costs and discuss: (1) whether all future costs are relevant for decision making and (2) whether variable costs are always relevant and fixed costs are always irrelevant
-
Propose a mechanism for the following transformation: [H,SO,] Meo
-
Propose a plausible synthesis for each of the following transformations: a. b.
-
In each group of compounds below, select the most acidic compound: (a) (b) Z Z CI CI CI
-
What are the benefits and potential risks factors for undertaking derivative strategies compared to cash transactions
-
business law A partner may actively compete with the partnership True False
-
A company provided the following data: Selling price per unit $80 Variable cost per unit $45 Total fixed costs $490,000 How many units must be sold to earn a profit of $122,500?

Study smarter with the SolutionInn App


