Consider two separate gas containers at the following conditions: How is the pressure in container B related
Question:
Consider two separate gas containers at the following conditions:
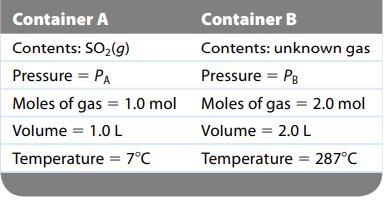
How is the pressure in container B related to the pressure in container A?
Transcribed Image Text:
Container A Contents: SO₂(g) Pressure = PA Container B Contents: unknown gas Pressure = PB of gas Moles of gas Volume = 2.0 L Temperature = 287°C Moles of gas = 1.0 mol Moles Volume = 1.0 L Temperature = 7°C = 2.0 mol
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 30% (10 reviews)
They are related by a ratio The pressure in container B is twice the pressure as in container A So ...View the full answer

Answered By

Brian Otieno
I'm Brian , an experienced professional freelancer with countless hours of success in freelancing many subjects in different disciplines. Specifically, I have handled many subjects and excelled in many disciplines. I have worked on many Computer Science projects and have been able to achieve a lot in that field. Additionally, I have handled other disciplines like History, Humanities, Social Sciences, Political science, Health care and life science, and Religion / Theology. My experience generally in these subjects has made me able to deliver high-quality projects in a very timely fashion. I am very reliable at my job and will get the work done in time, no matter what. In Addition, I have managed to ensure that the work meets my client's expectations and does not cause an error. I am a hard-working and diligent person who is highly responsible for everything I do. Generally, Freelancing has made me more accountable for doing my job. Additionally, I have had a passion for writing for the last seven years in this field.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
Use the definition of the definite integral to justify the property where f is continuous and c is a real number. x = c [" f(x) dx, S"cf(x) dx a
-
A gas in a container had a measured pressure of 57 kPa. Calculate the pressure in units of atm and mmHg.
-
Consider the following gas container equipped with a movable piston. a. By what factor (increase by 1, decrease by 1.5, etc.) would you change the pressure if you wanted the volume to change from...
-
Research about the competitive and comparative advantage of the Argentina in terms of physical and human resources and how it is making use of these resources for international trade purpose. 3....
-
Matilda and John Perry owned property that was damaged by fire. The Perrys were insured against property damage caused by fire under an insurance policy issued by AFRD Insurance Company of Canada....
-
An article in Technometrics by S. C. Narula and J. F. Wellington [Prediction, Linear Regression, and a Minimum Sum of Relative Errors (1977, Vol. 19)] presents data on the selling price and annual...
-
6 Give examples of how an information system can affect at least two of the forces in Porters model and so affect the competitiveness of a business.
-
(a) As a source of long-term financing, what are the major advantages of bonds over common stock? (b) What are the major disadvantages in using bonds for long-term financing?
-
9. value 2.00 points Bad Debts Expense using a % of Net Credit Sales Superior Company has provided you with the following information before any year-end adjustments: Net credit sales are $135,500...
-
Through an analysis of the English court's rulings in recent environmental claims brought against Shell Plc (Okpabi and Others v Royal Dutch Shell plc UKSC 2021 3; and ClientEarth v Shell plc and...
-
Concentrated hydrogen peroxide solutions are explosively decomposed by traces of transition metal ions (such as Mn or Fe): What volume of pure O 2 (g), collected at 27 C and 746 torr, would be...
-
A 5.0-L flask contains 0.60 g O 2 at a temperature of 22 C. What is the pressure (in atm) inside the flask?
-
4. What is a characteristic of an option? a Gives the holder the right but not the obligation to buy or sell b Negotiated with a counterparty c Covers a stream of future payments d Must be settled...
-
A popular theory is that presidential candidates have an advantage if they are taller than their main opponents. Listed are heights (in centimeters) of randomly selected presidents along with the...
-
Cash Flows Horiz Analysis Horiz Analysis Vertic Analysis Vertic Analysis from Oper Inc St Bal St Inc St Bal Sheet Ratios Requirement Prepare the cash flows from operations section of R. Ashburn...
-
Rudy Gandolfi owns and operates Rudy's Furniture Emporium Incorporated. The balance sheet totals for assets, liabilities, and stockholders' equity at August 1, 2022, are as indicated. Described here...
-
The brand manager for a brand of toothpaste must plan a campaign designed to increase brand recognition. He wants to first determine the percentage of adults who have heard of the brand. How many...
-
Pulse rates of women are normally distributed with a mean of 77.5 beats per minute and a standard deviation of 11.6 beats per minute. Answer the following questions. What are the values of the mean...
-
A dietitian at General Hospital wants a patient to have a meal that has 47 grams (g) of protein, 58 g of carbohydrates, and 630 milligrams (mg) of calcium. The hospital food service tells the...
-
Chapter 9 Stock Valuation at Ragan Engines Input area: Shares owned by each sibling Ragan EPS Dividend to each sibling Ragan ROE Ragan required return Blue Ribband Motors Corp. Bon Voyage Marine,...
-
Determine the electron configuration for each of the following atoms: a. Carbon b. Oxygen c. Boron d. Fluorine e. Sodium f. Aluminum
-
In each case, identify the more stable anion. Explain why it is more stable. (a) (b) (c) vs. N. vs. -zo
-
Atropine, extracted from the plant Atropa belladonna, has been used in the treatment of bradycardia (low heart rate) and cardiac arrest. Draw the enantiomer of atropine: CH 0= -
-
BUS 280 Week 9 Assignment This week the assignment is about financial management. You will prepare a Cash Flow Statement for Clark's Sporting Goods and then you will calculate ratios for Sam's Paint...
-
Ayayai Restaurant's gross payroll for April is $46,800. The company deducted $2,551 for CPP$739 for Eland $9,026 for income taxes from the employeeschequesEmployees are paid monthly at the end of...
-
44. Dryer Companys policy is to keep 25% of the next month's sales in ending inventory. If Dryer meets its ending inventory policy at the end of April and sales are expected to be 24,000 units in May...

Study smarter with the SolutionInn App


