Determine the phases that are present and the compositions for each phase in Cu-85 wt% Ag at
Question:
Determine the phases that are present and the compositions for each phase in Cu-85 wt% Ag at 800°C.
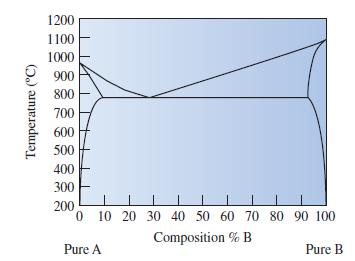
Transcribed Image Text:
Temperature (°C) 1200 1100 1000 900 800 700 600 500 400 300 200 0 10 20 30 40 50 60 70 80 90 100 Composition % B Pure A Pure B
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 69% (13 reviews)
ANSWER At 800 C the phases present are liquid an...View the full answer

Answered By

Aketch Cindy Sunday
I am a certified tutor with over two years of experience tutoring . I have a passion for helping students learn and grow, and I firmly believe that every student has the potential to be successful. I have a wide range of experience working with students of all ages and abilities, and I am confident that I can help students succeed in school.
I have experience working with students who have a wide range of abilities. I have also worked with gifted and talented students, and I am familiar with a variety of enrichment and acceleration strategies.
I am a patient and supportive tutor who is dedicated to helping my students reach their full potential. Thank you for your time and consideration.
0.00
0 Reviews
10+ Question Solved
Related Book For 

The Science And Engineering Of Materials
ISBN: 9781305076761
7th Edition
Authors: Donald R. Askeland, Wendelin J. Wright
Question Posted:
Students also viewed these Engineering questions
-
Determine the phases that are present and the compositions for each phase in Cu-55 wt% Ag at 600C. Data Form Problem 11-23 Consider a Pb-70% Sn alloy. Determine (a) If the alloy is hypoeutectic or...
-
Cite the phases that are present and the phase compositions for the following alloys: (a) 90 wt% Zn-10 wt% Cu at 400(C (750(F) (b) 75 wt% Sn-25 wt% Pb at 175(C (345(F) (c) 55 wt% Ag-45 wt% Cu at...
-
Cite the phases that are present and the phase compositions for the following alloys: (a) 90 wt% Zn-10 wt% Cu at 400C (750F) (b) 75 wt% Sn-25 wt% Pb at 175C (345F) (c) 55 wt% Ag-45 wt% Cu at 900C...
-
Consider a static (one-period), closed economy with one representative consumer, one rep- resentative firm, and a government. The level of capital K and government expenditures G in the economy are...
-
For the given of Problem 11, indicate: (a) How much would an interest arbitrageur earn if the foreign currency were at a forward premium of 1 percent per year? (b) What would happen if the foreign...
-
Marcus is the HR manager for United Airlines, an Illinois-based company. One of his employees has recently become disabled and is unable to fulfill the essential functions of his current position,...
-
Ticker Services began operations in 2011 and maintains long-term investments in available-for-sale securi ties. The year-end cost and fair values for its portfolio of these investments follow....
-
Steve and Marie Venturini were involved in the operation of Steves Sizzling Steakhouse in Carlstadt, New Jersey, from the day their parents opened it in the 1930s. By the 1980s, Steve, Marie, and her...
-
Hi, i need help with this information. One thing i purchased recently was the iphone 12 Pro Max. me, perferably i like the max iphones In today's world, many people use the Internet, smartphones, and...
-
Use the k NN algorithm to classify the new data in the Excel file Credit Approval Decisions Coded using only credit score and years of credit history as input variables.
-
A Pb-Sn alloy contains 45% and 55% at 100C. Determine the composition of the alloy. Is the alloy hypoeutectic or hypereutectic?
-
A Pb-Sn alloy contains 23% primary a and 77% eutectic microconstituent immediately after the eutectic reaction has been completed. Determine the composition of the alloy.
-
Our bank will finance the product expansion project with at a loan interest rate of 10 percent. Make sure the projects cash flow estimates include this interest expense. Do you agree or disagree?...
-
Sketch plane / intersecting plane K. Then draw a line & in plane J that intersects plane Kat a single point. A X C B D E
-
Use a graphing utility to verify any five of the graphs that you drew by hand in Exercises 126. Data from exercise 1-26 1. x + 2y = 8 3. x2y> 10 2. 3x6y 12 4. 2xy > 4
-
The following information pertains to Porter Company for 2011. Ending inventory consisted of 30 units. Porter sold 320 units at \(\$ 30\) each. All purchases and sales were made with cash. Required...
-
In Problems 7780, use a numerical integration routine on a graphing calculator to find the area bounded by the graphs of the indicated equations over the given interval (when stated). Compute answers...
-
Solar Heating, Inc., had the following transactions for 2011: Required a. Determine the quantity and dollar amount of inventory at the end of the year, assuming Solar Heating Inc. uses the FIFO cost...
-
On January 1, 2013, Sienna acquired all the shares of Danon for $160,000. The financial statements of the two entities at December 31, 2013, contained the following information: Additional...
-
Assume today is the 21st of February. Using the information below, FT Extract, answer the following questions (parts i and ii). You work for a US company that is due to receive 250 million in June...
-
(a) Using only K sp from Table 6-3, calculate how many moles of Ca(OH) 2 will dissolve in 1.00 L of water. (b) How will the solubility calculated in part (a) be affected by the K 1 reaction in Table...
-
From the following equilibrium constants, calculate the equilibrium constant for the reaction HO,CCO,H 2H* + C,0.
-
Assuming complete dissociation of the salts, calculate the ionic strength of (a) 0.2 mM KNO 3 ; (b) 0.2 mM Cs 2 CrO 4 ; (c) 0.2 mM MgCl 2 plus 0.3 mM AlCl 3 .
-
The Balance Sheet has accounts where the accountant must make estimates. Some situations in which estimates affect amounts reported in the balance sheet include: Allowance for doubtful accounts....
-
Alado fis istirmerfs Tat likifond 205L [ridont inip lanod whadtinion? hingend is antan Qultit foer avdeed Divdasit errem yodichiders Etexlpoges Getmare nelp
-
The limitation on the deduction of business interest does not apply to non-corporate taxpayers. course hero True or False explain?

Study smarter with the SolutionInn App


