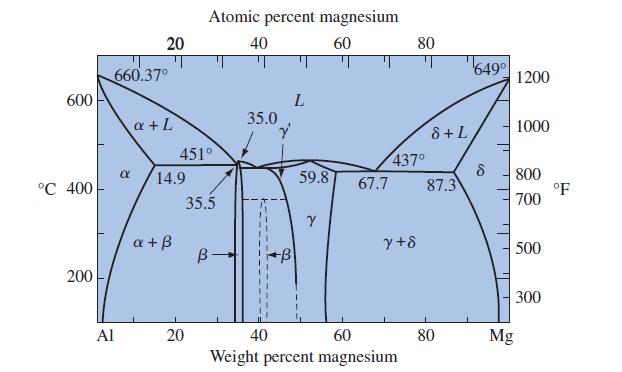
Examine Figure 12-10 and state the phases that will be present in each stage if this process
Question:
Examine Figure 12-10 and state the phases that will be present in each stage if this process path is followed: (500°C, 20 wt% Mg) to (500°C, 80 wt% Mg) to (200°C, 80 wt% Mg).
Transcribed Image Text:
600 °C 400 200 Al 20 660.37° α a + L 14.9 a + ß 451° Atomic percent magnesium 40 60 20 35.5 B- 35.0 Y 322 L 59.8 Y 67.7 437⁰ y+8 80 40 60 Weight percent magnesium 8 + L 87.3 80 649⁰ 8 Mg 1200 1000 800 700 500 300 °F
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 90% (10 reviews)
The phases include i High temperature melt which wil...View the full answer

Answered By

Brian Otieno
I'm Brian , an experienced professional freelancer with countless hours of success in freelancing many subjects in different disciplines. Specifically, I have handled many subjects and excelled in many disciplines. I have worked on many Computer Science projects and have been able to achieve a lot in that field. Additionally, I have handled other disciplines like History, Humanities, Social Sciences, Political science, Health care and life science, and Religion / Theology. My experience generally in these subjects has made me able to deliver high-quality projects in a very timely fashion. I am very reliable at my job and will get the work done in time, no matter what. In Addition, I have managed to ensure that the work meets my client's expectations and does not cause an error. I am a hard-working and diligent person who is highly responsible for everything I do. Generally, Freelancing has made me more accountable for doing my job. Additionally, I have had a passion for writing for the last seven years in this field.
0.00
0 Reviews
10+ Question Solved
Related Book For 

The Science And Engineering Of Materials
ISBN: 9781305076761
7th Edition
Authors: Donald R. Askeland, Wendelin J. Wright
Question Posted:
Students also viewed these Engineering questions
-
What polarization mechanism will be present in (a) alumina; (b) copper; (c) silicon; and (d) barium titanate?
-
Figure P19.62 shows a thermodynamic process followed by 120 mg of helium. a. Determine the pressure (in atm), temperature (in C), and volume (in cm 3 ) of the gas at points 1, 2, and 3. Put your...
-
Examine Figure 8-12 to see that competitive firm C is not producing at all. Explain the reason why the profit maximizing output level for firm C is at q, = 0. What would happen to total industry cost...
-
Clara Hughes, who is pushing 5 0 , has medaled both in speedskating and road cycling ( and showing no signs of slowing down ) completed a training event where she biked 5 0 km east, stopped and rode...
-
Explain how the capital expenditures budget is different from the expense budget.
-
What is the return on the IO and PO at prepayment rates of 25 percent and 30 percent?
-
(a) In a troubled debt situation, why might the creditor grant concessions to the debtor? (b) What type of concessions might a creditor grant the debtor in a troubled debt situation? (c) What is...
-
The investments of Harry and Belinda have done well through the years. While the cash portion of their portfolio has risen to $16,000, it is earning a minuscule 1 percent in a money market account;...
-
takeAssignmentMain.do?invoker=&takeAssignmentSessionLocator=&inprogress=false Calculator Selected accounts with amounts omitted are as follows Work in Process Aug. 1 Balance 263,150 Aug. 31 31 Direct...
-
The Rock Glen House B&B is operated as a sole proprietorship and had the following income and expenses for the year: Room rental income $137,900 Vending machine income 2,000 Advertising expense...
-
Draw the eutectoid portion of the Fe- Fe 3 C phase diagram. Be sure to indicate all of the compositions and temperatures and write the relevant reaction.
-
Little Books Inc. recently reported $3 million of net income. Its EBIT was $6 million, and its tax rate was 40%. What was its interest expense? [Hint: Write out the headings for an income statement...
-
The products of combustion enter a nozzle of a jet engine at a total pressure of 18 lbf/in 2, and a total temperature of 1200 F. The atmospheric pressure is 6.75 lbf/in 2. The nozzle is convergent,...
-
As a project manager it is important to utilize the right tool at the right time. When it comes to managing quality on projects, this is no exception. Identify three 'Total Quality Tools' that you...
-
Describe 2 change models that you could use to create change in an organization. Choose 1 of the models that you think would be most successful in an organization, and analyze reasons why you chose...
-
During the current year, Rothchild, Inc., purchased two assets that are described as follows. Heavy Equipment Purchase price, $375,000. Expected to be used for 10 years, with a residual value at the...
-
Regarding the Mozilla case, assume that Communities of Practice start to arise spontaneously around topics that are related to the visualizations in the Portal at Mozilla. What do you think is the...
-
Regarding Issues That Affect Recruitment, how would you proceed as the assistant superintendent for human resources in a school district that is experiencing a shortage of qualified applicants for...
-
Refer to the figure and find the volume generated by rotating the given region about the specified line. R 3 about OA y B(1, 1) C(0, 1) R2 y= Vr R3 Rr A(1, 0)
-
Which of the following streaming TV devices does not involve use of a remote controller? A) Google Chromecast B) Apple TV C) Amazon Fire TV D) Roku
-
Using estimates of rainfall, evaporation, and water consumption, the town engineer developed the following model of the water volume in the reservoir as a function of time where V is the water volume...
-
The volume V and paper surface area A of a conical paper cup are given by where r is the radius of the base of the cone and h is the height of the cone. a. By eliminating h, obtain the expression for...
-
A torus is shaped like a doughnut. If its inner radius is a and its outer radius is b, its volume and surface area are given by a. Create a user-defined function that computes V and A from the...
-
BUS 280 Week 9 Assignment This week the assignment is about financial management. You will prepare a Cash Flow Statement for Clark's Sporting Goods and then you will calculate ratios for Sam's Paint...
-
Ayayai Restaurant's gross payroll for April is $46,800. The company deducted $2,551 for CPP$739 for Eland $9,026 for income taxes from the employeeschequesEmployees are paid monthly at the end of...
-
44. Dryer Companys policy is to keep 25% of the next month's sales in ending inventory. If Dryer meets its ending inventory policy at the end of April and sales are expected to be 24,000 units in May...

Study smarter with the SolutionInn App


