Suppose K = 4.5 10 -3 at a certain temperature for the reaction If it is
Question:
Suppose K = 4.5 × 10-3 at a certain temperature for the reaction
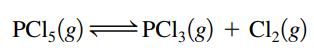
If it is found that the concentration of PCl5 is twice the concentration of PCl3, what must be the concentration of Cl2 under these conditions?
Transcribed Image Text:
PC15 (8) PC13 (8) PC13(g) + Cl₂(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 84% (13 reviews)
Solution The concentratio...View the full answer

Answered By

James Warinda
Hi! I’m James Otieno and I'm an experienced professional online tutor with countless hours of success in tutoring many subjects in different disciplines. Specifically, I have handled general management and general business as a tutor in Chegg, Help in Homework and Trans tutor accounts.
I believe that my experience has made me the perfect tutor for students of all ages, so I'm confident I can help you too with finding the solution to your problems. In addition, my approach is compatible with most educational methods and philosophies which means it will be easy for you to find a way in which we can work on things together. In addition, my long experience in the educational field has allowed me to develop a unique approach that is both productive and enjoyable.
I have tutored in course hero for quite some time and was among the top tutors awarded having high helpful rates and reviews. In addition, I have also been lucky enough to be nominated a finalist for the 2nd annual course hero award and the best tutor of the month in may 2022.
I will make sure that any student of yours will have an amazing time at learning with me, because I really care about helping people achieve their goals so if you don't have any worries or concerns whatsoever you should place your trust on me and let me help you get every single thing that you're looking for and more.
In my experience, I have observed that students tend to reach their potential in academics very easily when they are tutored by someone who is extremely dedicated to their academic career not just as a businessman but as a human being in general.
I have successfully tutored many students from different grades and from all sorts of backgrounds, so I'm confident I can help anyone find the solution to their problems and achieve
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
The following equilibrium pressures were observed at a certain temperature for the reaction N2(g) + 3H2(g) 2NH3(g) PNH3 = 3.1 10-2 atm PN2 = 8.5 10-1 atm PH2 = 3.1 10-3 atm Calculate the value...
-
It is found that a 6.00-m segment of a long string contains four complete waves and has a mass of 180 g. The string is vibrating sinusoidally with a frequency of 50.0 Hz and a peak-to-valley distance...
-
Suppose that following the CME it is found that at an altitude of 2500 m the electric field has magnitude 1300 N/C and at an altitude of 1500 m the magnitude is 3600 N/C. In both cases the direction...
-
Consider the pooled t variable Tp from part (b) of the previous exercise. a. Use this t variable to obtain a pooled t confidence interval formula for 1 2 . b. The article Effect of Welding on a...
-
What is a supply chain, and how is it different from a channel of distribution?
-
Anthropologists can estimate the birthrate of an ancient society by studying the age distribution of skeletons found in ancient cemeteries. The numbers of skeletons found at two such sites, as...
-
Differentiate between sensitivity and scenario analyses. What advantage does scenario analysis have over sensitivity analysis? AppendixLO1
-
On January 3, 2014, Mega Limited purchased 3,000 shares (30%) of the common shares of Sonja Corp. for $438,000. The following information is provided about the identifiable assets and liabilities of...
-
I want a quick solution without explanation Question 39 Not yet answered Marked out of 1.00 P Flag question Which approach will help in developing a strong trust between a sales manager and sales...
-
As an airplane?s brakes are applied, the nose wheel exerts two forces on the end of the landing gear as shown. Determine the horizontal and vertical components of reaction at the pin C and the force...
-
At a certain temperature, K = 9.1 10 -4 for the reaction Calculate the concentrations of Fe 3+ , SCN - , and FeSCN 2+ in a solution that is initially 2.0 M FeSCN 2+ . 3+ FeSCN2+ (aq) Fe+ (aq) + SCN-...
-
Old-fashioned smelling salts consist of ammonium carbonate, (NH 4 ) 2 CO 3 . The reaction for the decomposition of ammonium carbonate is endothermic. Would the smell of ammonia increase or decrease...
-
Shah is a retail company specializing in mens hats. Its budget director prepared the list of expected operating expenses that follows. All items are paid when the expenses are incurred except sales...
-
1. State the difference between lists and tuples in Python programming. 2. Explain why Python is an Interpreted Language
-
1. How does Python handle memory? 2. Python's ternary operators: how do they work? 3. How is Python's multithreading implemented
-
In a relational database, explain the difference between Inner join & Outer join. Provide an example query for each and describe the result set produced by each query.
-
Use a graphing utility to solve equation. Express the solution(s) rounded to two decimal places. 6 sin x - e x = 2, x > 0
-
At 31 December 20X9, the end of the annual reporting period, the accounts of Huron Company showed the following: a. Sales revenue for 20X9, $ 2,950,000, of which one- quarter was on credit. b....
-
When 0.575 g of monosodium glutamate (MSG) is dissolved in 10.0 mL of water and placed in a sample cell 10.0 cm in length, the observed rotation at 20C (using the D line of sodium) is +1.47....
-
When 0.095 g of cholesterol is dissolved in 1.00 mL of ether and placed in a sample cell 10.0 cm in length, the observed rotation at 20C (using the D line of sodium) is -2.99. Calculate the specific...
-
When 1.30 g of menthol is dissolved in 5.00 mL of ether and placed in a sample cell 10.0 cm in length, the observed rotation at 20C (using the D line of sodium) is +0.57. Calculate the specific...
-
Your firm is planning to invest in an automated packaging plant. Harburtin Industries is an all - equity firm that specializes in this business. Suppose Harburtin ' s equity beta is 0 . 8 7 , the...
-
Ned Allen opened a medical practice in Los Angeles, California, and had the following transactions during the month of January. (Click the icon to view the January transactions.) Journalize the...
-
do you need more information or are you working on this? Irene Watts and John Lyon are forming a partnership to which Watts will devote one- half time and Lyon will devote full time. They have...

Study smarter with the SolutionInn App


