The mechanism for the gas-phase reaction of nitrogen dioxide with carbon monoxide to form nitric oxide and
Question:
The mechanism for the gas-phase reaction of nitrogen dioxide with carbon monoxide to form nitric oxide and carbon dioxide is thought to be
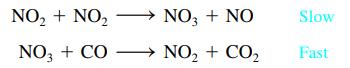
Write the rate law expected for this mechanism. What is the overall balanced equation for the reaction?
Transcribed Image Text:
NO₂ + NO₂ NO3 + CO → NO3 + NO NO₂ + CO₂ Slow Fast
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 91% (12 reviews)

Answered By

Aun Ali
I am an Associate Member of Cost and Management Accountants of Pakistan with vast experience in the field of accounting and finance, including more than 17 years of teaching experience at university level. I have been teaching at both undergraduate and post graduate levels. My area of specialization is cost and management accounting but I have taught various subjects related to accounting and finance.
5.00+
13+ Reviews
32+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
Nitrogen dioxide, NO2, decomposes upon heating to form nitric oxide and oxygen according to the following equation: 2NO2(g) 2NO(g) + O2(g) At the beginning of an experiment, the concentration of...
-
Like nickel, iron reacts with carbon monoxide to form a compound having the formula M(CO)n that obeys the 18-electron rule. What is the value of n in the formula Fe(CO)n?
-
Nitrogen dioxide reacts with carbon monoxide by the overall equation NO2(g)+ CO(g) NO(g) + CO2(g) At a particular temperature, the reaction is second order in NO2 and zero order in CO. The rate...
-
What is the advantage of using computing to simulate an automobile crash test as opposed to actually staging a crash?
-
Explain the many-to-many communication model and why it is important for marketers today.
-
Suppose that population increases at a fixed rate n. For this model economy, verify that the horizontal intercept of the feasible set line is equal to y and that the vertical intercept of the...
-
Its stock price is $14 per share and it has 5 million shares outstanding. The firms total capital is $125 million and it finances with only debt and common equity. What is its debt-to-capital ratio?...
-
Why cant we pay our shareholders a dividend? Shouts your new boss at Polar Opposites, This income statement you prepared for me says we earned $5million in our first year! You recently prepared the...
-
What is your average speed in miles per hour and in feet per second if you travel a mile in 11 minutes? Click the icon to view the table of USCS length measurements
-
In early July 2012, Dr. Elaine Matthews separated from her husband of some years. She maintained full custody of the couple's only child, a seven-year-old girl. Since May 1, 1998, Dr. Matthews had...
-
One reason suggested for the instability of long chains of silicon atoms is that the decomposition involves the transition state shown below: The activation energy for such a process is 210 kJ/mol,...
-
A certain substance, initially present at 0.0800 M, decomposes by zero-order kinetics with a rate constant of 2.50 10 -2 mol/L ? s. Calculate the time (in seconds) required for the system to reach a...
-
Give the structures of the polymers formed from the monomers given below, showing at least two repeat units. For each polymer identify the following: i. The repeat unit ii. The type of linkage...
-
Go to: https://www.instagram.com/ryderseyewear/ on your desktop, laptop, or mobile (or a combination of all 3). You are the new Social Media Marketing Manager for Ryders Eyewear. You've been asked...
-
As leaders, it is very important that we have the ability to assess our own motivation and the motivation of others around us. It is also important to recognize the key factors involved in...
-
At the end of this exam, you will find Article 1 - " How Companies Can Prepare for a Long Run of High Inflation ". Please read the article and, when necessary, consult additional sources and the...
-
You can develop your capabilities as a manger by better understanding different ways of motivating and rewarding employees. You can also better prepare for your own career by better understanding the...
-
Topic: Project Malasakit of Kara David https://projectmalasakit.org/ What is the pros and cons of these alternative courses of the action below: Strengthen the internal organization via promoting it...
-
Use a calculator to solve equation on the interval 0 2. Round answers to two decimal places. csc = -3
-
6 (a) Briefly develop a mathematical model of the behaviour of a copper-twisted pair cable (b) Derive the magnetic energy from: w given that: K + w, where the - - k symbols have their usual meaning...
-
Propose a plausible mechanism for each of the following hydrolysis reactions: (a) (b) (c) (d) EtO OEt * * + 2 ELOH (b) N. * .N'
-
Propose a plausible mechanism for the reaction below: [H2SO4] N- - . -N.
-
As shown above, methenamine is hydrolyzed in aqueous acid to produce formaldehyde and ammonia. Draw a mechanism showing formation of one molecule of formaldehyde (the remaining five molecules of...
-
exercise 4-7 (Algo) Effects of transactions on income statement LO P2
-
Compute the value of ordinary bonds under the following circumstances assuming that the coupon rate is 0.06:(either the correct formula(s) or the correct key strokes must be shown here to receive...
-
A tax-exempt municipal bond has a yield to maturity of 3.92%. An investor, who has a marginal tax rate of 40.00%, would prefer and an otherwise identical taxable corporate bond if it had a yield to...

Study smarter with the SolutionInn App


