One reason suggested for the instability of long chains of silicon atoms is that the decomposition involves
Question:
One reason suggested for the instability of long chains of silicon atoms is that the decomposition involves the transition state shown below:
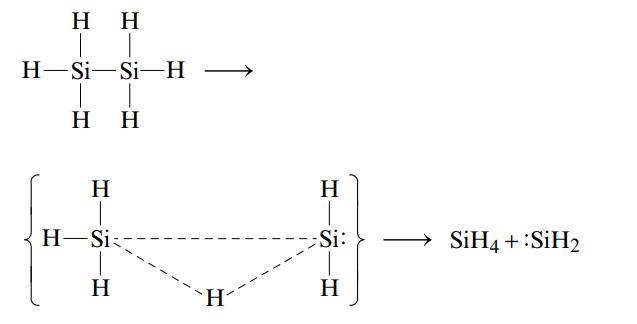
The activation energy for such a process is 210 kJ/mol, which is less than either the Si-Si or the Si-H bond energy. Why would a similar mechanism not be expected to play a very important role in the decomposition of long chains of carbon atoms as seen in organic compounds?
Transcribed Image Text:
Η Η H-Si-Si-H H Η H Η H H-Si; Η H H Si: Η SiH4 + :SiH2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 84% (13 reviews)
It is because carbon has a more stable sp hybrid sorbital t...View the full answer

Answered By

Brian Otieno
I'm Brian , an experienced professional freelancer with countless hours of success in freelancing many subjects in different disciplines. Specifically, I have handled many subjects and excelled in many disciplines. I have worked on many Computer Science projects and have been able to achieve a lot in that field. Additionally, I have handled other disciplines like History, Humanities, Social Sciences, Political science, Health care and life science, and Religion / Theology. My experience generally in these subjects has made me able to deliver high-quality projects in a very timely fashion. I am very reliable at my job and will get the work done in time, no matter what. In Addition, I have managed to ensure that the work meets my client's expectations and does not cause an error. I am a hard-working and diligent person who is highly responsible for everything I do. Generally, Freelancing has made me more accountable for doing my job. Additionally, I have had a passion for writing for the last seven years in this field.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:
Students also viewed these Engineering questions
-
Why can international trade not be expected to be an engine of growth for today's developing nations? In what ways can international trade still play a very important supportive role for development...
-
Mentors can play a very important role in a persons career. Please describe how a person could go about finding a mentor.
-
Organic compounds containing radioactive atoms can be used to follow the biosynthesis of molecules in cells and organisms. In these experiments, the amount of radioisotope in proteins derived from...
-
According to Dr. Grant, creatives are people who ________. A. Are procrastinators B. Act on multiple ideas C. Plan out each detail D. Are highly confident with no doubts about their success
-
What is buzz? How do marketers practice buzz building?
-
Consider an investment project for which the cash flow pattern repeats itself every four years indefinitely, as shown in the accompanying figure. At an interest rate of 12% compounded annually,...
-
DuPONT ANALYSIS Doublewide Dealers has an ROA of 10%, a 2% profit margin, and an ROE of 15%. What is its total assets turnover? What is its equity multiplier? AppendixLO1
-
Learning curve, cumulative average-time learning model Global Defense manufactures radar systems. It has just completed the manufacture of its first newly designed system, RS-32. Manufacturing data...
-
sams farms just paid a dividend of 4.50 on its stock. The growth rate in dividend is expected to be a constant 3% per year indefinitely. Investors require a 13% return on the stock for the first 3...
-
Presented below is selected information related to the financial instruments of Dawson Company at December 31, 2010. This is Dawson Companys first year of operations. Instructions (a) Dawson elects...
-
The rate constant for the gas-phase decomposition of N 2 O 5 , has the following temperature dependence: Make the appropriate graph using these data, and determine the activation energy for this...
-
The mechanism for the gas-phase reaction of nitrogen dioxide with carbon monoxide to form nitric oxide and carbon dioxide is thought to be Write the rate law expected for this mechanism. What is the...
-
Analogue Technology has preferred stock outstanding that pays a $9 annual dividend. It has a price of $76. What is the required rate of return (yield) on the preferred stock?
-
In the introduction to "The Five Sexes," Anne Fausto-Sterling writes that she had to "invent conventions - s/he and his/her - to denote someone who is clearly neither male nor female or who is...
-
Select a product described as one of the "Biggest Product Flops" of 2019 that you will bring back to the market. To, you will need to engage in some research to understand why the product failed to...
-
Breaking the Bank Case Questions (video found at: http://www.pbs.org/wgbh/pages/frontline/breakingthebank/view/?utm_campaign=viewpage &utm_medium=grid&utm_source=grid) 1) To what extent were the...
-
Please answer in full and write legibly. Suppose Alice has taken 7 classes college, and her current GPA is 3.48 (assume for simplicity that all courses carry the same number of credits). Answer the...
-
F. Explain how to overcome two potential biases (e.g., prejudice, discrimination) using culturally competent strategies that will help improve stakeholder communication. G. Explain how to mitigate...
-
Use a calculator to solve equation on the interval 0 2. Round answers to two decimal places. 5 tan + 9 = 0
-
Chicago Company sold merchandise to a customer for $1,500 cash in a state with a 6% sales tax rate. The total amount of cash collected from the customer was $558. $600. $642. $636. Nevada Company...
-
Treatment of catechol with formaldehyde in the presence of an acid catalyst produces a compound with molecular formula C 7 H 6 O 2 . Draw the structure of this product. HO Catechol
-
Predict the major product(s) for each reaction below. (a) (b) (c) 1) LAH 2) , 1) PhMgBr 2) H,0
-
Starting with cyclopentanone and using any other reagents of your choosing, identify how you would prepare each of the following compounds: (a) (b) (c) (d)
-
For anOld Country Links, Incorporated, produces sausages in three production departments Mixing , Casing and Curing, and Packaging. In the Mixing Department, meats are prepared, ground and mixed with...
-
A manufacturing firm uses a predetermined manufacturing overhead rate to allocate overhead to individual jobs, based on machine hours required. At the beginning of 2 0 1 9 , the firm expected to...
-
An investor wants to purchase a zero coupon bond from Timberlake Industries today. The bond will mature in exactly 5.00 years with a redemption value of $1,000. The investor wants a 12.00% annual...

Study smarter with the SolutionInn App


