Develop expressions for h, u, s, P r , and v r for an ideal gas whose
Question:
Develop expressions for h, u, s°, Pr, and vr for an ideal gas whose cop is given by
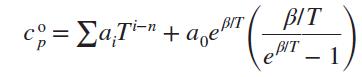
where ai, a0, n, and β are empirical constants.
Transcribed Image Text:
c = Ea,T- BIT eNT - 1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 77% (9 reviews)
Expressions for h u s P r and v r for an ideal gas whose c o p is ...View the full answer

Answered By

Utsab mitra
I have the expertise to deliver these subjects to college and higher-level students. The services would involve only solving assignments, homework help, and others.
I have experience in delivering these subjects for the last 6 years on a freelancing basis in different companies around the globe. I am CMA certified and CGMA UK. I have professional experience of 18 years in the industry involved in the manufacturing company and IT implementation experience of over 12 years.
I have delivered this help to students effortlessly, which is essential to give the students a good grade in their studies.
3.50+
2+ Reviews
10+ Question Solved
Related Book For 

Thermodynamics An Engineering Approach
ISBN: 9781259822674
9th Edition
Authors: Yunus Cengel, Michael Boles, Mehmet Kanoglu
Question Posted:
Students also viewed these Engineering questions
-
An ideal gas whose adiabatic exponent equals goes through a cycle consisting of two isochoric and two isobaric lines. Find the efficiency of such a cycle, if the absolute temperature of the gas rises...
-
An ideal gas whose adiabatic exponent equals is expanded so that the amount of heat transferred to the gas is equal to the decrease of its internal energy. Find: (a) The molar heat capacity of the...
-
One mole of an ideal gas whose adiabatic exponent equals undergoes a process in which the gas pressure relates to the temperature as p = aTa, where a and a are constants. Find: (a) The work performed...
-
On September 30 of the current year, Silver Fox Corporation files for bankruptcy. At the time, it estimates that the total FMV of its assets is $725,000, whereas the total amount of its outstanding...
-
A study was conducted to compare the incubation periods and fledgling periods (in days) of different bird species. Sample data are given below for randomly selected species. a. Do the incubation and...
-
DE 19-13 Jell's manufactures women's plastic sandals. Suppose the company's March records C include the following items. What is Jell's total manufacturing overhead cost in March? Ink for printing...
-
How do the goals set for a marketing program in the planning phase relate to the evaluation phase of the strategic marketing process? Answer: The planning phase goals or objectives are used as the...
-
City Place Movie Theaters has four employees and pays them on an hourly basis. During the week beginning June 24 and ending June 30, 2019, these employees worked the hours shown below. Information...
-
HOUT (The following information apples to the questions displayed below.) Simon Company's year-end balance sheets follow Current Y 1 YAO 2 Yes Ago At December 31 Assets Cash Accounts receivable, net...
-
On a late November morning in 2012, a boutique investment bank in Japan approached Mr. Takuya Saito, founder and CEO of Saito Solar, about his interest in selling the rm. Even though selling the rm...
-
Reconsider Prob. 1279. Determine the exergy destruction associated with the process. Assume T0 = 30C. Data From Reconsider Prob. 1279: Propane is compressed isothermally by a pistoncylinder device...
-
For ideal gases, the development of the constant-volume specific heat yields Prove this by using the definitions of pressure and temperature, T = (u/s)v and P = (u/v)s. = 0 du.
-
The book value of shares issued in exchange for control rights should be used as the basis of measuring the initial cost. True/False
-
Suppose a company bases its hourly rates on the number of customers per hour. The hourly rate the company charges is given by two functions where = g(2) 4, g(3) = 2, 9(4) = 3 and f(2) = 6, f(3) = 3,...
-
Which statements about insurance are true? 1- Insurance protects against the the worst-case scenario. All rational people want to buy insurance. 2- Insurance costs money, and therefore always...
-
need step by step instruction about creating this: in NX12 PART NAME: BRACKET ALL FILLETS R .313 ALL ROUNDS R .625 2X .500 1/500 2.875 9.500 4750 2875 $500 3.000 750 GENTERED IN OBJECT 2.375
-
8. Convert the angle - 7t from radian measure into degree measure. Show some work. 4
-
4. Variance Analysis. (CPA, adapted) The H. G. Company uses a standard cost system in accounting for the cost of one of its products. < The Budget is based on normal capacity of monthly production of...
-
For the part shown, answer the following questions with regard to the cylindrical boss. (a) What are the maximum and minimum diameters allowed for the boss? (b) What is the effect of the position...
-
Rowland Textile Inc. manufactures two products: sweatshirts and T-shirts. The manufacturing process involves two activities: cutting and sewing. Expected overhead costs and cost drivers are as...
-
A textile plant requires 4 kg/s of saturated steam at 2 MPa, which is extracted from the turbine of a cogeneration plant. Steam enters the turbine at 8 MPa and 500C at a rate of 11 kg/s and leaves at...
-
A thermoelectric refrigerator is powered by a 12-V car battery that draws 3 A of current when running. The refrigerator resembles a small ice chest and is claimed to cool nine canned drinks, 0.350-L...
-
Thermoelectric coolers that plug into the cigarette lighter of a car are commonly available. One such cooler is claimed to cool a 12-oz (0.771-lbm) drink from 78 to 38oF or to heat a cup of coffee...
-
When preparing government-wide financial statements, the modified accrual based governments funds are adjusted. Please show the adjustments (in journal entry form with debits and credits) that would...
-
I need help finding the callable price and call value
-
On 31 October 2022, the owner took goods for his son as a birthday gift. The cost price of the goods was R15 000

Study smarter with the SolutionInn App


