For ideal gases, the development of the constant-volume specific heat yields Prove this by using the definitions
Question:
For ideal gases, the development of the constant-volume specific heat yields
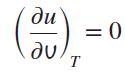
Prove this by using the definitions of pressure and temperature, T = (∂u/∂s)v and P = −(∂u/∂v)s.
Transcribed Image Text:
ди = 0 du.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 58% (12 reviews)
It is to be proven by using the definitions of ...View the full answer

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Thermodynamics An Engineering Approach
ISBN: 9781259822674
9th Edition
Authors: Yunus Cengel, Michael Boles, Mehmet Kanoglu
Question Posted:
Students also viewed these Engineering questions
-
For ideal gases, the development of the constant-pressure specific heat yields Prove this by using the definitions of pressure and temperature, T = (u/s)v and P = - (u/v)s. ah aP
-
Plss answer the highlighted number SERVATION OF MASS AND ENERGY unknown flow rate; however, the water temperature increases from 13C to 24 C. Also, it is known that 1 kg of water will absorb 4.2 kJ...
-
14 Thermodynamics and Thermochemistry . The reaction, MgO(s) + C(s) Mg(s) + CO(g ) 18 The entropy change associated with the conversion of 1 kg of ice at 273 K to water vapours at 383 K is for which...
-
Route Canal Shipping Company has the following schedule for aging of accounts receivable: AGE OF RECEIVABLES APRIL 30, 2001 a. Fill in column (4) for each month. b. If the firm had $1,440,000 in...
-
The paired data below consist of weights (in kilograms) of discarded paper and sizes of the households. Paper Household size 2 1.09 3.43 4.33 4.00 3.96 3.16 3.10 5.18
-
DE 19-12 Turn to Exhibit 19-12 (page 767). If direct material purchases were $35,000 rather than $27,000, what would be the cost of direct materials used and the cost of goods manufactured? (Other...
-
(a) Using Netflix as an example, explain how a mission statement gives it a strategic direction. (b) Create a mission statement for your own career. LO.1
-
Floyd's Bumpers has distribution centers in Lafayette, Indiana: Charlotte, North Carolina; Los Angeles, California; Dallas, Texas; and Pittsburgh, Pennsylvania. Each distribution center carries all...
-
The unadjusted and adjusted trial balance for Blossom Company are as follows. Prepare the income statement for the year ended August 31. Three parts to this problem other two are hidden BLOSSOM...
-
The night manager of Dixie Transportation Service, who had no accounting background, prepared the following balance sheet for the company at February 28, 2011. The dollar amounts were taken directly...
-
Develop expressions for h, u, s, P r , and v r for an ideal gas whose c o p is given by where a i , a 0 , n, and are empirical constants. c = Ea,T- BIT eNT - 1
-
Estimate the c p of nitrogen at 300 kPa and 400 K, using (a) The relation in Prob. 1291, Data From Q#91: Consider an infinitesimal reversible adiabatic compression or expansion process. By taking s =...
-
What is the difference between the way operators are implemented in C++ and Ruby?
-
The following graph shows a market supply curve in orange and a market demand curve in blue. Suppose there is an increase in demand and an increase in supply. Adjust the following graph to reflect...
-
AICPA and PCAOB auditing standards address the confirmation of accounts receivables. Under the currently effective standards, what are the circumstances under which confirmation of accounts...
-
Maphitha Limited produces a single type of a product. The company uses an actual costing system. The following information has been taken from the company's production and sales records for the month...
-
Master Budget was made for annual sale of 100,000 units @10 per unit. Actual sales figures were 80,000 units with a sales revenues of 840,00. The standard cost sheet indicated a variable...
-
Sales of a product was estimated at 80,000 pieces annually with a rate of 6 pu. Its variable mfg. costs are 2.50 pu with S&D and general expenses related to product is 59,000 on annual basis. Its...
-
For the part shown, the ideal position of the cylindrical boss is located with the basic dimensions of 100 and 50. These basic dimensions are measured from which of the following? (Select one.) i....
-
1. Using the information from Problem 16-4B, prepare a statement of cash flows for Lim Garden Supplies Inc. using the direct method of presenting cash flows from operating activities. 2. How does Lim...
-
It is proposed to run a thermoelectric generator in conjunction with a solar pond that can supply heat at a rate of 7 106 kJ/h at 90oC. The waste heat is to be rejected to the environment at 22oC....
-
A typical 200-m2 house can be cooled adequately by a 3.5-ton air conditioner whose COP is 4.0. Determine the rate of heat gain of the house when the air conditioner is running continuously to...
-
Consider a steady-flow Carnot refrigeration cycle that uses refrigerant-134a as the working fluid. The maximum and minimum temperatures in the cycle are 30 and - 20oC, respectively. The quality of...
-
What is Coke's average ownership percentage in its equity method investments? Goodwill is 7000 Calculate the firm's current ratio (current assets/current liabilities). Calculate the current ratio...
-
John has to choose between Project A and Project B, which are mutually exclusive. Project A has an initial cost of $30,000 and an internal rate of return of 16 percent. Project B has an initial cost...
-
Complete the table below, for the above transactions

Study smarter with the SolutionInn App


