On a summer day in New Orleans, Louisiana, the pressure is 1 atm: the temperature is 32C;
Question:
On a summer day in New Orleans, Louisiana, the pressure is 1 atm: the temperature is 32°C; and the relative humidity is 95 percent. This air is to be conditioned to 24°C and 60 percent relative humidity. Determine the amount of cooling, in kJ, required and water removed, in kg, per 1000 m3 of dry air processed at the entrance to the system.
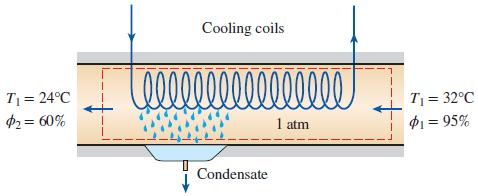
Cooling coils 00000000 T = 24°C $2= 60% T = 32°C P1 = 95% 1 atm Condensate
Step by Step Answer:
Air is cooled and dehumidified at constant pressure The amount of water removed from the air and the ...View the full answer



Thermodynamics An Engineering Approach
ISBN: 9781259822674
9th Edition
Authors: Yunus Cengel, Michael Boles, Mehmet Kanoglu
Related Video
In this video, the concept of pressure has been explained. The bottle is filled up with water, remove the pipe, and now you can see the clear flow of water from the holes due to liquid pressure.You can see that the second layer is holding the weight of the first layer and the third layer holding the weight of both upper layers, that’s why liquid flows out wider from the third as compared to the first two.At the first hole, water doesn’t roll along the walls; it flows out at an angle.At the second hole, you’ll see that the water flows out at a wider angle as compared to the first hole.At the third hole, we can see that the flow of water is much wider/greater than the other holes.This proves that the pressure at the bottom of the bottle is much more than the pressure at the top layers. P = ????gh
Students also viewed these Engineering questions
-
Reconsider Prob. 1482. How far will the temperature of the humid air have to be reduced to produce the desired dehumidification? Data From Reconsider Prob. 1482: On a summer day in New Orleans,...
-
During a summer day in El Paso, Texas, outdoor air is at 1 atm, 40C, and 20 percent relative humidity. Water at 20C is evaporated into this air to produce air at 25C and 80 percent relative humidity....
-
During a summer day in Phoenix, Arizona, the air is at 1 atm, 110oF, and 15 percent relative humidity. Water at 70oF is evaporated into this air to produce air at 75oF and 80 percent relative...
-
The following items were shown on the balance sheet of Herman Corporation on December 31, 2010: Stockholders Equity Paid-In Capital Capital Stock Common stock , $5 par value, 360,000 shares...
-
An observed frequency distribution is as follows: Number of successes 0 1 2 3 Frequency 89 133 52 26 a. Assuming a binomial distribution with n = 3 and p = 1/3, use the binomial probability...
-
P 5-7 Consolidation workpapers (upstream sales, noncontrolling interest) Pam Corporation purchased a 90 percent interest in Sun Corporation on December 31, 2015, for $2,700,000 cash, when Sun had...
-
A decrease in excess reserves implies in the ability of banks to lend.
-
What is the present worth of a series of equal quarterly payments of $3000 that extends over a period of 8 years if the interest rate is 10% compounded monthly?
-
In its income statement for the year ended December 31, 2022, Windsor, Inc. reported the following condensed data. Salaries and wages expenses $427,800 Loss on disposal of plant assets Cost of goods...
-
What type of experimental design would you recommend in each of the following cases? Suggest in some detail how you would design each study: (a) A test of three methods of compensation of factory...
-
Atmospheric air from the inside of an automobile enters the evaporator section of the air conditioner at 1 atm, 27C, and 50 percent relative humidity. The air returns to the automobile at 10C and 90...
-
Repeat Prob. 1479 for a total pressure of 88 kPa for air. Data From Repeat Prob. 1479: Air enters a 40-cm-diameter cooling section at 1 atm, 32C, and 70 percent relative humidity at 120 m/min. The...
-
a. On the same axes sketch the graphs of y = x 2 (x 2) and y = 2x x 2 . b. By solving a suitable equation find the points of intersection of the two graphs.
-
7. Chicago Corp. obtained the following information from the Raw Materials Inventory account and purchasing records for the first quarter of the current year: Beginning Raw Materials Ending Raw...
-
Suppose that i t =6% (n=1), and that future short term interest rates (n=1) for the next 3 years (starting next year) are expected to be: 4%, 2%, 2%. Suppose that the liquidity premium is zero for...
-
Mechanical Vibrations HW Use the modal analysis and numerical integration to compute and plot the time response of the system, which has the equations of motion [8 0 01 (1) 48 -12 01(x1 0 0 8 02-12...
-
Submit excel file with graph and exchange rate analysis. FOREIGN EXCHANGE RATESTHE YEN FOR DOLLARS. The Federal Reserve System Web site, www.federalreserve.gov/releases/H10/hist , provides historical...
-
Part 1: There are many types of communication styles used in the workplace. Choose what you think is your leadership style: north, south, east, or west. Click The Leadership Compass Self-Assessment...
-
An AISI 4142 steel Q&T at 800F exhibits S yt = 235 kpsi, S yc = 285 kpsi, and f = 0.07. For the given state of plane stress, (a) Determine the factor of safety, (b) Plot the failure locus and the...
-
A red card is illuminated by red light. What color will the card appear? What if its illuminated by blue light?
-
An ideal-gas mixture consists of 2 kmol of N2 and 6 kmol of CO2. The mass fraction of CO2 in the mixture is (a) 0.175 (b) 0.250 (c) 0.500 (d) 0.750 (e) 0.875
-
An ideal-gas mixture consists of 2 kmol of N2 and 4 kmol of CO2. The apparent gas constant of the mixture is (a) 0.215kJ/kgK (b) 0.225kJ/kgK (c) 0.243kJ/kgK (d) 0.875kJ/kgK (e) 1.24kJ/kgK
-
A rigid tank is divided into two compartments by a partition. One compartment contains 3 kmol of N2 at 400 kPa and the other compartment contains 7 kmol of CO2 at 200 kPa. Now the partition is...
-
Break-Even Sales and Sales to Realize Income from Operations For the current year ending October 31, Yentling Company expects fixed costs of $537,600, a unit variable cost of $50, and a unit selling...
-
You buy a stock for $35 per share. One year later you receive a dividend of $3.50 per share and sell the stock for $30 per share. What is your total rate of return on this investment? What is your...
-
Filippucci Company used a budgeted indirect-cost rate for its manufacturing operations, the amount allocated ($200,000) is different from the actual amount incurred ($225,000). Ending balances in the...

Study smarter with the SolutionInn App


