Reconsider Prob. 1482. How far will the temperature of the humid air have to be reduced to
Question:
Reconsider Prob. 14–82. How far will the temperature of the humid air have to be reduced to produce the desired dehumidification?
Data From Reconsider Prob. 14–82:
On a summer day in New Orleans, Louisiana, the pressure is 1 atm: the temperature is 32°C; and the relative humidity is 95 percent. This air is to be conditioned to 24°C
and 60 percent relative humidity. Determine the amount of cooling, in kJ, required and water removed, in kg, per 1000 m3 of dry air processed at the entrance to the system.
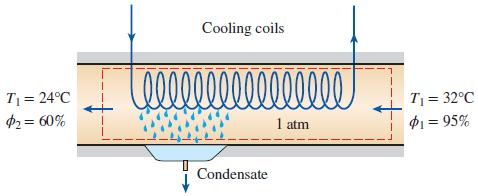
Cooling coils 0000000 T = 32°C P1 = 95% T = 24°C 2= 60% 1 atm Condensate
Step by Step Answer:
The humid air of the previous problem is reconsidered The exit temperature of the air to pr...View the full answer



Thermodynamics An Engineering Approach
ISBN: 9781259822674
9th Edition
Authors: Yunus Cengel, Michael Boles, Mehmet Kanoglu
Related Video
In this video, the concept of pressure has been explained. The bottle is filled up with water, remove the pipe, and now you can see the clear flow of water from the holes due to liquid pressure.You can see that the second layer is holding the weight of the first layer and the third layer holding the weight of both upper layers, that’s why liquid flows out wider from the third as compared to the first two.At the first hole, water doesn’t roll along the walls; it flows out at an angle.At the second hole, you’ll see that the water flows out at a wider angle as compared to the first hole.At the third hole, we can see that the flow of water is much wider/greater than the other holes.This proves that the pressure at the bottom of the bottle is much more than the pressure at the top layers. P = ????gh
Students also viewed these Engineering questions
-
On a summer day in New Orleans, Louisiana, the pressure is 1 atm: the temperature is 32C; and the relative humidity is 95 percent. This air is to be conditioned to 24C and 60 percent relative...
-
If the system of Prob. 14-122E is operated as an adiabatic system and the air produced by this system has a relative humidity of 70 percent, what is the temperature of the air produced? Prob. 14-122E...
-
A power company located in southern Alabama wants to predict the peak power load (i.e., Y, the maximum amount of power that must be generated each day to meet demand) as a function of the daily high...
-
Nancy has active modified adjusted gross income before passive losses of $75,000. She has a loss of $5,000 on a rental property she actively manages. How much of the loss is she allowed to take...
-
In a recent year, customers of Victoria Fridge and Stove (which is open 7 days a week) returned 146 appliances. If the frequencies of returns on different days conform to a Poisson distribution, they...
-
P 5-6 Upstream and downstream sales, 90 percent owned Justin Bhd is a 90 percent-owned company of Epik Bhd and was acquired in 2011, when the book value of Justin Bhds net identifiable assets were...
-
Decreasing the reserve requirement should lead to in the supply of money.
-
Blue Marlin Company is considering the purchase of new equipment for its factory. It will cost $250,000 and have a $50,000 salvage value in five years. The annual net income from the equipment is...
-
The following information relates to Toolworks Ltd.s inventory transactions during the month of October. Units Cost/Unit Amount Oct. 1 Beginning inventory 2,480 $ 12.70 $ 31,496 5 Sale 350 11...
-
Consider two different machines, with two different instruction sets, both of which have a clock rate of 200 MHz. The following measurements are recorded on the two machines running a given set of...
-
Atmospheric air from the inside of an automobile enters the evaporator section of the air conditioner at 1 atm, 27C, and 50 percent relative humidity. The air returns to the automobile at 10C and 90...
-
Repeat Prob. 1479 for a total pressure of 88 kPa for air. Data From Repeat Prob. 1479: Air enters a 40-cm-diameter cooling section at 1 atm, 32C, and 70 percent relative humidity at 120 m/min. The...
-
Explain why a tenancy at sufferance is not really a tenancy at all, and why a tenancy at will may not really be a true lease either.
-
Question 1 [40 marks] (a) Table 1 present experimental data related to the absorbance of two compounds over a range of concentration, in a UV-Vis cell with path length I = 1.0 cm. From this table:...
-
i. The following table presents data on wholesale gas prices for the major capital cities in the Eastern-half of Australia, from 2011-12 to 2022-23. Use this data to construct a single, time-series...
-
Problem 1 Using the same Fourier-Method approach as used in lecture, consider a beam loaded as shown below. 290 -q. Cos 280 x Shane land V-280 Distributed load w = =-80 . Cos[X] a. What are the...
-
Think about a Floor Warden training program for that company - and write me another email (attached here as a Word document) as if I were the leader of your organization to tell me about the...
-
A particle travels around the curve shown, following ? = ? 0 . 2 ? ? , ?with ? ( ? ) = 0 . 5 ? 2 rad. At the moment ? = ? , ?determine the speed and acceleration of the particle. ? = , ? ? ? = , ? ?...
-
An AISI 4142 steel Q&T at 800F exhibits S yt = 235 kpsi, S yc = 285 kpsi, and f = 0.07. For the given state of plane stress, (a) Determine the factor of safety, (b) Plot the failure locus and the...
-
Prove the result that the R 2 associated with a restricted least squares estimator is never larger than that associated with the unrestricted least squares estimator. Conclude that imposing...
-
Repeat Prob. 13-9 by replacing N2 by O2. In Prob. 13-9 A gas mixture has the following composition on a mole basis: 60 percent N2 and 40 percent CO2. Determine the gravimetric analysis of the...
-
Using Amagat's law, show that for a real-gas mixture of k gases, where Z is the compressibility factor. z,- , =D1 Z. i=1
-
An ideal-gas mixture whose apparent molar mass is 20 kg/kmol consists of N2 and three other gases. If the mole fraction of nitrogen is 0.55, its mass fraction is (a) 0.15 (b) 0.23 (c) 0.39 (d) 0.55...
-
Los datos de la columna C tienen caracteres no imprimibles antes y despus de los datos contenidos en cada celda. En la celda G2, ingrese una frmula para eliminar cualquier carcter no imprimible de la...
-
Explain impacts of changing FIFO method to weighted average method in inventory cost valuations? Explain impacts of changing Weighted average method to FIFO method in inventory cost valuations?...
-
A perpetuity makes payments starting five years from today. The first payment is 1000 and each payment thereafter increases by k (in %) (which is less than the effective annual interest rate) per...

Study smarter with the SolutionInn App


