Repeat Prob. 128 for helium. Data From Q#8: Consider air at 350 K and 0.75 m 3
Question:
Repeat Prob. 12–8 for helium.
Data From Q#8:
Consider air at 350 K and 0.75 m3/kg. Using Eq. 12–3, determine the change in pressure corresponding to an increase of
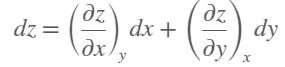
(a) 1 percent in temperature at constant specific volume
(b) 1 percent in specific volume at constant temperature
(c) 1 percent in both the temperature and specific volume.
Transcribed Image Text:
dz dz dx + dy = %3D дх. y ду,
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 63% (11 reviews)
Helium at a specified temperature and specific volume is considered ...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Thermodynamics An Engineering Approach
ISBN: 9781259822674
9th Edition
Authors: Yunus Cengel, Michael Boles, Mehmet Kanoglu
Question Posted:
Students also viewed these Engineering questions
-
Repeat Problem 12-5 for helium. Problem 12-5 Consider air at 350 K and 0.75 m3/kg. Using Eq. 12-3, determine the change in pressure corresponding to an increase of (a) 1 percent in temperature at...
-
Consider air at 350 K and 0.75 m3/kg. Using Eq. 12-3, Determine the change in pressure corresponding to an increase of (a) 1 percent in temperature at constant specific volume. (b) 1 percent in...
-
Consider air at 400 K and 0.90 m3/kg. Using Eq. 123, determine the change in pressure corresponding to an increase of (a) 1 percent in temperature at constant specific volume, (b) 1 percent in...
-
Jacomo Companys output for the current period was assigned a $300,000 standard direct materials cost. The direct materials variances included a $44,000 favorable price variance and a $6,000 favorable...
-
Find the regression equation; unless the problem suggests otherwise, let the first variable be the independent (x) variable. Caution: When finding predicted values, be sure to follow the prediction...
-
P19-7B Consider the Discount Gas proposed entry into e-commerce in Problem 19-6B. Steve Pronai revises his estimates of the benefits from the new system's lower labor costs. He now thinks there is a...
-
Discuss how an organization assesses where it is now and where it seeks to be. LO.1
-
The dollar cost of debt for Coval Consulting, a U.S. research firm, is 7.5%. The firm faces a tax rate of 30% on all income, no matter where it is earned. Managers in the firm need to know its yen...
-
Question 5 of 5 Belinda borrowed $ 2 0 , 0 0 0 at simple interest rate of 6 . 2 0 % p . a . from her parents to start a business. At the end of 3 months, she paid them $ 6 , 0 0 0 and $ 2 , 0 0 0 at...
-
I am going to fly to San Francisco on December 8th and return home on December 11th. My travel agent tells me that I could buy a one-way ticket today to San Francisco for $350 or a round-trip ticket...
-
Consider air at 350 K and 0.75 m 3 /kg. Using Eq. 123, determine the change in pressure corresponding to an increase of (a) 1 percent in temperature at constant specific volume (b) 1 percent in...
-
Show how you would evaluate T, v, u, a, and g from the thermodynamic function h = h(s, P).
-
How has the severity of the business cycle varied over time in the United States? What is the Great Moderation?
-
The Role of Leadership in Shaping Organizational Culture Recent research stated that [c]ompanies with an established organizational culture that includes strong capabilities for change, commitment to...
-
Unscheduled absences by clerical and production workers are an important cost in many companies. Reducing the rate of absenteeism is, therefore, an important goal for a company's human relations...
-
Many of the largest tech firms, including Google, Apple, Amazon, and Microsoft, have spent hundreds of millions of dollars to improve their information technology infrastructure. Now, these companies...
-
In the business sense, a product refers to a commodity available for purchase, encompassing both services and tangible or intangible items. It may exist in physical, virtual, or cyber forms. Every...
-
Data Exploration and Multiple Linear Regression (MLR) using SAS. The "College" data set contains the statistics for many US Colleges from 1995 issue of US News and World Report. It has 777...
-
The tax researcher must be able to find descriptions of tax treaties to solve certain tax problems. List different locations where a tax researcher might find a tax treaty.
-
Annual dividends of ATTA Corp grew from $0.96 in 2005 to $1.76 in 2017. What was the annual growth rate?
-
Using EES (or other) software, determine how much the thermal efficiency of the cycle in Prob. 10-22 would change if there were a 50 kPa pressure drop across the boiler.
-
The net work output and the thermal efficiency for the Carnot and the simple ideal Rankine cycles with steam as the working fluid are to be calculated and compared. Steam enters the turbine in both...
-
A binary geothermal power plant uses geothermal water at 160°C as the heat source. The cycle operates on the simple Rankine cycle with isobutane as the working fluid. Heat is transferred to the...
-
How to solve general ledger cash balance chapter 9 assignment 5
-
On 31 July 2018, Sipho bought 1 000 ordinary shares in ABC Ltd at a cost of R2 750. On 31 December 2018 the company made a 1 for 10 bonus issue. On 31 March 2019, Sipho sold 300 shares for R800. What...
-
If you purchase a $1000 par value bond for $1065 that has a 6 3/8% coupon rate and 15 years until maturity, what will be your annual return? 5.5% 5.9% 5.7% 6.1%

Study smarter with the SolutionInn App


