The equation of state of a gas is given by where a and b are constants. Use
Question:
The equation of state of a gas is given by
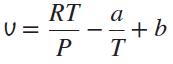
where a and b are constants. Use this equation of state to derive an equation for the Joule-Thomson coefficient inversion line.
Transcribed Image Text:
RT U = a + b T %3D
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (12 reviews)
The equation of state of a gas is given by An equation for the JouleThomson coefficient inv...View the full answer

Answered By

Somshukla Chakraborty
I have a teaching experience of more than 4 years by now in diverse subjects like History,Geography,Political Science,Sociology,Business Enterprise,Economics,Environmental Management etc.I teach students from classes 9-12 and undergraduate students.I boards I handle are IB,IGCSE, state boards,ICSE, CBSE.I am passionate about teaching.Full satisfaction of the students is my main goal.
I have completed my graduation and master's in history from Jadavpur University Kolkata,India in 2012 and I have completed my B.Ed from the same University in 2013. I have taught in a reputed school of Kolkata (subjects-History,Geography,Civics,Political Science) from 2014-2016.I worked as a guest lecturer of history in a college of Kolkata for 2 years teaching students of 1st ,2nd and 3rd year. I taught Ancient and Modern Indian history there.I have taught in another school in Mohali,Punjab teaching students from classes 9-12.Presently I am working as an online tutor with concept tutors,Bangalore,India(Carve Niche Pvt.Ltd.) for the last 1year and also have been appointed as an online history tutor by Course Hero(California,U.S) and Vidyalai.com(Chennai,India).
4.00+
2+ Reviews
10+ Question Solved
Related Book For 

Thermodynamics An Engineering Approach
ISBN: 9781259822674
9th Edition
Authors: Yunus Cengel, Michael Boles, Mehmet Kanoglu
Question Posted:
Students also viewed these Engineering questions
-
The van der Waals equation of state, an approximate representation of the behavior of gases at high pressure, is given by Where a and b are constants having different values for different gases. (In...
-
The equation of state of a gas is given as (P + 10/ 2) = RuT, where the units of and P are m3/kmol and kPa, respectively. Now 0.5 kmol of this gas is expanded in a quasi-equilibrium manner from 2 to...
-
The equation of state of a gas is given as v-(P + 10/ v-2) = 5 RuT, where the units of v- and P are m3/kmol and kPa, respectively. Now 0.2 kmol of this gas is expanded in a quasi equilibrium manner...
-
What must be the beta of a portfolio with E( rP ) = 18%, if rf = 6% and E (rM) = 14%?
-
Find the regression equation; unless the problem suggests otherwise, let the first variable be the independent (x) variable. Caution: When finding predicted values, be sure to follow the prediction...
-
E19-3 Suppose Target incurred the following costs at its Charleston, South Carolina. store. Research to determine whether store should add Payment to consultant for advice on location of new a travel...
-
What is the difference between an organizations business and its goals? Answer: An organizations business describes the clear, broad, underlying industry or market sector of an organizations...
-
A fire recently destroyed a substantial portion of Manley Companys production capacity. It will be many months before capacity can be restored. During this period, demand for the firms products will...
-
Question 2 Question 2 of 1 5 6 . 6 6 points Sedita Inc. is working on its cash budget for July. The budgeted beginning cash balance is $ 4 6 , 0 0 0 . Budgeted cash receipts total $ 1 7 5 , 0 0 0 and...
-
In a pretest, data on Nike were obtained from 45 respondents. These data are given in the following table, which gives the usage, sex, awareness, attitude, preference, intention, and loyalty toward...
-
What is the most general equation of state for which the Joule-Thomson coefficient is always zero?
-
What is the enthalpy departure?
-
What are the arguments in favor of using trade barriers to enforce labor and environmental standards? Assess each argument.
-
Problem 2-26 (Static) Complete the balance sheet using cash flow data LO 2-2, 2-3, 2-5, 2-6 Following is a partially completed balance sheet for Epsico Incorporated at December 31, 2022, together...
-
Consider the following potential events that might have occurred to Global Conglomerate on December30, 2018. For eachone, indicate which line items inGlobal's balance sheet would be affected and by...
-
An epidemiologist plans to conduct a survey to estimate the percentage of women who give birth. How many women must be surveyed in order to be 95% confident that the estimated percentage is in error...
-
Jamonit Ltd is a non-group employer which paid wages of $136,000 in the Northern Territory during March 2021. The company does not pay wages in any other state. Calculate the payroll tax payable in...
-
Following is a partially completed balance sheet for Epsico Inc. at December 31, 2019, together with comparative data for the year ended December 31, 2018. From the statement of cash flows for the...
-
The diameter of a cylindrical boss is dimensioned 25 0.2. A position control is used to control the basic location of the boss. Specify the diameters allowed for the position tolerance zone if the...
-
Revol Industries manufactures plastic bottles for the food industry. On average, Revol pays $76 per ton for its plastics. Revol's waste-disposal company has increased its waste-disposal charge to $57...
-
Why is the combined gas-steam cycle more efficient than either of the cycles operated alone?
-
The gas-turbine portion of a combined gas-steam power plant has a pressure ratio of 16. Air enters the compressor at 300 K at a rate of 14 kg/s and is heated to 1500 K in the combustion chamber. The...
-
Consider a combined gas-steam power plant that has a net power output of 450 MW. The pressure ratio of the gas-turbine cycle is 14. Air enters the compressor at 300 K and the turbine at 1400 K. The...
-
Read the following and then answer the questions below:September 12: A Brisbane business offers by letter to sell 500 tyres to a New Zealand company. The Brisbane company does not specify a method of...
-
Fred returns home from work one day to discover his house surrounded by police. His wife is being held hostage and threatened by her captor. Fred pleads with the police to rescue her and offers...
-
Would like you to revisit one of these. Consideration must be clear and measurable.if you can't measure it then how can you show it has / has not been done?How can you sue someone for breach of...

Study smarter with the SolutionInn App


