Work can be produced by passing the vapor phase of a two-phase substance stored in a tank
Question:
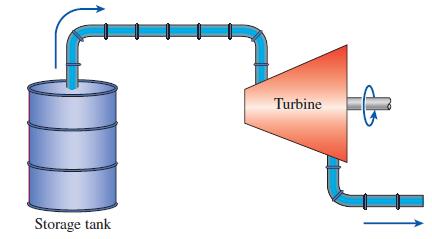
Work can be produced by passing the vapor phase of a two-phase substance stored in a tank through a turbine as shown in Fig. P7–198E. Consider such a system using R-134a, which is initially at 80°F, and a 10-ft3 tank that initially is entirely filled with liquid R-134a. The turbine is isentropic, the temperature in the storage tank remains constant as mass is removed from it, and the R-134a leaves the turbine at 10 psia. How much work will be produced when the half of the liquid mass in the tank is used?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Thermodynamics An Engineering Approach
ISBN: 9781259822674
9th Edition
Authors: Yunus Cengel, Michael Boles, Mehmet Kanoglu
Question Posted:





