Air at 50 psia and 90 F flows at the rate of 1.6 lb m /s through
Question:
Air at 50 psia and 90 F flows at the rate of 1.6 lbm/s through an insulated ideal turbine. If the air delivers 11.5 hp to the turbine blades, at what temperature does the air leave the turbine?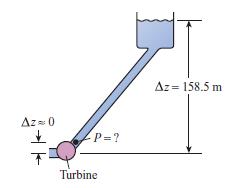
Transcribed Image Text:
Δz = 0 k 1 με P=? Turbine Δ== 158.5 m
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
Given Inlet pressure P1 50 psia Inlet temperature T1 90 F Mass flow rate m 16 lbms Power output W 11...View the full answer

Answered By

Rodrigo Louie Rey
I started tutoring in college and have been doing it for about eight years now. I enjoy it because I love to help others learn and expand their understanding of the world. I thoroughly enjoy the "ah-ha" moments that my students have. Interests I enjoy hiking, kayaking, and spending time with my family and friends. Ideal Study Location I prefer to tutor in a quiet place so that my students can focus on what they are learning.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Thermodynamics Concepts And Applications
ISBN: 9781107179714
2nd Edition
Authors: Stephen R. Turns, Laura L. Pauley
Question Posted:
Students also viewed these Engineering questions
-
Air at 50 psia and 90 F flows through an expander (like a turbine) at the rate of 1.6 lbm/s to an exit pressure of 14.7 psia. (a) What is the minimum temperature attainable at the expander exit if...
-
An insulated 40-ft3 rigid tank contains air at 50 psia and 120F. A valve connected to the tank is now opened, and air is allowed to escape until the pressure inside drops to 25 psia. The air...
-
A rigid tank contains 20 lbm of air at 50 psia and 80F. The air is now heated until its pressure doubles. Determine (a) The volume of the tank and (b) The amount of heat transfer.
-
Use trigonometry/parallelogram law to determine the resultant force (magnitude and direction from the positive x-axis) of the two forces. Lunits units 30 Problem 1/2 27 units
-
Prepare journal entries to record the following transactions for a retail store. Assume a perpetual inventory system. Apr. 2 Purchased merchandise from Johns Company under the following terms: $5,900...
-
What is lot traceability? Why is it important to safety related products?
-
Refer to the completely randomized ANOVA design conducted in Example 10.4. Are the assumptions required for the test approximately satisfied? LO4
-
Harwell Company manufactures automobile tires. On July 15, 2011, the company sold 1,000 tires to the Nixon Car Company for $50 each. The terms of the sale were 2/10, n/30. Harwell uses the net method...
-
Problem 5. On November 1, 2017, US Pelican Company entered into a 90 day forward contract of 200,000 pounds to hedge a commitment to purchase special equipment on February 1, 2018 from a British firm...
-
During a year, Teri's monthly sales compensation ranged between $12,000 and $16,000 per month and units sold ranged between 1,600 and 2,400 units for those same months. Required: Use the high-low...
-
A 200-ft 3 /min flow of air at 14.7 psia and 60 F enters a fan with negligible inlet velocity. The fan discharge duct has a cross-sectional area of 3 f t2 . The process across the fan is isentropic...
-
Consider the situation described in Example 8.74; however, imagine that the turbine is now located at one-half z1 and that the discharge pipe is extended down to z 2 (= 0), the original discharge...
-
Radium-226 is a radioactive isotope that decays exponentially at a rate of 0.0428% per year. The amount of radium-226, R, remaining after t years can be found by the formula R(t) = R 0 e -0.000428t ,...
-
To create 3 scenarios (positive, neutral and negative) for the development of the restaurant. Include the following important factors in your assessments: border trade as one of the most important...
-
Description of market segment Aged between 34 and 58 Regular commuters Clerical or professional Income over $50K Moderately price-sensitive but may see higher price as an indicator of quality...
-
How has the job of the manager changed over time (since the publication of the Mintzberg article below)? What factors have contributed to shifts in the manager's role? What new roles are managers...
-
a. illustrate the fives modes within which an ethical leader can exercise authority b. Evaluate four factors related to interpersonal dimension that relate to unethical behaviour of leaders c. Assess...
-
Complete the following writing assignment: Analyze the attached 10_pages. Write about them, summarize what you read, and connect it to personal experiences. CHAPTER 15 Sexual Dysfunctions and...
-
Determine the residual molar entropies for molecular crystals of the following: a. 35 Cl 37 Cl b. CFCl 3 c. CF 2 Cl 2 d. CO 2
-
For the following arrangements, discuss whether they are 'in substance' lease transactions, and thus fall under the ambit of IAS 17.
-
A 1500 kN load was applied on two 20 m long and 500 mm diameter piles that were instrumented for measuring the load variation with depth. a. The variation of frictional resistance per unit area, f...
-
A 300 mm 450 mm plate was used in carrying out a plate loading test in a sand, where the plate settled 5 mm under the applied pressure of 250 kN/m 2 . a. What is the coefficient of subgrade reaction...
-
For a 2.0 m wide and 0.40 m thick beam on elastic foundation, determine the coefficient of subgrade reaction k using Eqs. (10.45) and (10.46), assuming the following parameters: E s = 30 MN/m 2 , E F...
-
Assume that the one-year interest rate in the US is 4% and in the Eurozone is 6%. According to interest rate parity (IRP), What should the one-year forward premium or discount of the euro be (use of...
-
Comparative financial statements for Weller Corporation, a merchandising company, for the year ending December 31 appear below. The company did not issue any new common stock during the year. A total...
-
Mrquered Mrquered

Study smarter with the SolutionInn App


