Determine the total apparent specific heat at constant pressure (c p,mix in kJ/kg K) for a
Question:
Determine the total apparent specific heat at constant pressure (cp,mix in kJ/kg · K) for a fuel–air reactant mixture containing 1 kmol CH4, 2.5 kmol O2, and 9.4 kmol N2 at 500 K and 1 atm. Use specific heat values for each species from Table E.1.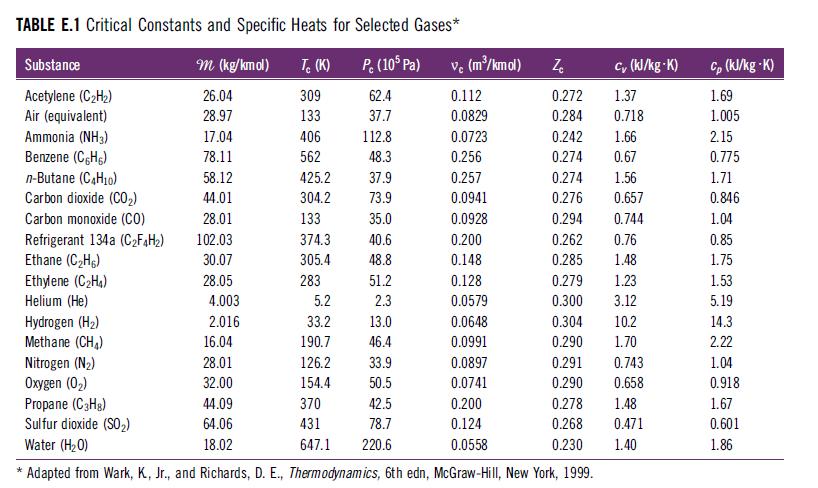
Transcribed Image Text:
TABLE E.1 Critical Constants and Specific Heats for Selected Gases* P. (105 Pa) 62.4 37.7 Substance Acetylene (C₂H₂) Air (equivalent) Ammonia (NH3) Benzene (CH) n-Butane (C4H10) Carbon dioxide (CO₂) Carbon monoxide (CO) * Refrigerant 134a (C₂F4H₂) Ethane (C₂Hg) Ethylene (C₂H4) Helium (He) m (kg/kmol) 26.04 28.97 17.04 78.11 58.12 44.01 28.01 102.03 30.07 28.05 4.003 2.016 Tc (K) 309 133 406 562 425.2 304.2 133 374.3 305.4 283 5.2 33.2 190.7 126.2 154.4 370 431 647.1 112.8 48.3 37.9 73.9 35.0 40.6 48.8 51.2 2.3 13.0 46.4 33.9 50.5 42.5 78.7 220.6 vc (m³/kmol) 0.112 0.0829 0.0723 0.256 0.257 0.0941 0.0928 0.200 0.148 0.128 0.0579 cy (kJ/kg-K) 1.37 0.718 0.242 1.66 0.274 0.67 0.274 1.56 0.276 0.657 0.294 0.744 0.262 0.76 0.285 1.48 Zc 0.272 0.284 0.279 1.23 0.300 3.12 Hydrogen (H₂) 0.0648 Methane (CH₂) 16.04 0.0991 Nitrogen (N₂) 28.01 0.0897 Oxygen (0₂) 32.00 0.0741 Propane (C3H8) 44.09 0.200 Sulfur dioxide (SO₂) 64.06 0.124 Water (H₂O) 18.02 0.0558 Adapted from Wark, K, Jr., and Richards, D. E., Thermodynamics, 6th edn, McGraw-Hill, New York, 1999. 0.304 0.290 0.291 0.290 10.2 1.70 0.743 0.658 0.278 1.48 0.268 0.471 0.230 1.40 Cp (kJ/kg-K) 1.69 1.005 2.15 0.775 1.71 0.846 1.04 0.85 1.75 1.53 5.19 14.3 2.22 1.04 0.918 1.67 0.601 1.86
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
To determine the total apparent specific heat at constant pressure for the given fuelair mixture we ...View the full answer

Answered By

Joshua Marie Geuvara
I am an academic writer with over 5 years of experience. I write term papers, essays, dissertations, reports, and any other academic paper. My main objective is to produce a high-quality paper free from plagiarism and ensure a student scores an A+. Being a fluent English speaker, I have great communication skills that also enable me to produce excellent papers.
I am conversant with most academic referencing styles (APA, MLA, and Harvard).
You can trust me with your paper and expect nothing less than quality and excellent results. I look forward to meeting with you and, more importantly, developing something that will both make us happy and satisfied.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Thermodynamics Concepts And Applications
ISBN: 9781107179714
2nd Edition
Authors: Stephen R. Turns, Laura L. Pauley
Question Posted:
Students also viewed these Engineering questions
-
Determine the total apparent specific heat at constant volume (c v,mix in kJ/kgK) for a fuelair reactant mixture containing 1 kmol CH 4 , 2.5 kmol O 2 , and 9.4 kmol N 2 at 500 K and 1 atm. What heat...
-
A mixture of ideal gases contains 0.5 kmol of CO 2 , 2 kmol of O 2 , and 7 kmol of N 2 at 700 K. Determine the following quantities: A. The mole fraction of each constituent in the mixture. B. The...
-
Complete the following questions. Submit journal entries in an Excel Template, linked at the bottom of this page, and any written segments in Excels comments function. Do not submit two separate...
-
Format only cells with values greater than maxim 4 tab and select Red color from the pallet (bottom ro Format only cells with values less than minimum and select Yellow color from the pallet (bottom...
-
Obtain IRS Revenue Ruling 59 60 through the IRS web site. Read this Ruling and prepare a memo to your professor explaining how each of its eight valuation factors fits into the theory of business...
-
8. LO.7 Caramel Corporation has 5,000 shares of stock outstanding. In a qualifying stock redemption, Caramel distributes $145,000 in exchange for 1,000 of its shares. At the time of the redemption,...
-
Recuerde los segmentos meta de marketing que identific en el captulo 8 para su plan de marketing: 1 a) Identifique cul de las cuatro redes sociales descritas en el captulo sera ms til y b) justifique...
-
At the beginning of its fiscal year, Caf Med leased restaurant space from Crescent Corporation under a nine-year lease agreement. The contract calls for annual lease payments of $25,000 each at the...
-
Non-current assets are_______. Select one: a. always shown in the statement of financial position at their original cost b. never removed from the business c. never depreciated over their useful life...
-
The balance sheet data for Angel?s Countertops, Inc., at August 31, 2018, and September 30, 2018, follow: Requirement The following are three independent assumptions about the business during...
-
A 1-m 3 tank contains nitrogen at 30C and 500 kPa. In an isothermal process, CO 2 is forced into this tank until the pressure is 1000 kPa. What is the mass (kg) of each gas present at the end of this...
-
A 17.3-liter tank contains a mixture of argon, helium, and nitrogen at 298 K. The argon and helium mole fractions are 0.12 and 0.35, respectively. If the partial pressure of the nitrogen is 0.8 atm,...
-
In 1992, Donna Smith telephoned Clark, the manager of Penbridge Farms, in response to an advertisement Clark had placed in the July issue of the Emu Finder about the availability for sale of proven...
-
Question 37 Plantito Inc., produces potted plants. For next year, Pietro predicts that 45,000 units will be produced, with the following total costs: Direct materials Direct labor ? 80,000 Variable...
-
When you are to design a data transmission system, you have two key considerations to work with: data rate and distance, with emphasis placed on achieving the highest data rates over the longest...
-
How much work does a supermarket checkout attendant do on a can of soup he pushes 0.600 m horizontally with a force of 5.00 N? Express your answer in joules and kilocalories. 3 . (a) Calculate the...
-
Suppose in its income statement for the year ended June 30, 2022, The Clorox Company reported the following condensed data (dollars in millions). Salaries and wages expenses$460 Research and...
-
Consider the extensive form game show in the figure below. How many strategies does Player 2 have in this game? (2,2,1) b (2,4,2) 03 by 03 02 dz (4.2,0) (2.0.2) (0.3.4) (3,5,3) (3,1,2)
-
This chapter covered many C-C bond-forming reactions, including aldol reactions, Claisen condensations, and Michael addition reactions. Two or more of these reactions are often performed...
-
Element compound homogeneous mixture (heterogeneous mixture) 4) A piece of gold has a mass of 49.75 g. What should the volume be if it is pure gold? Gold has a density of 19.3 g/cm (3 points) D=m/v...
-
Determine the value of the stoichiometric coefficients for the combustion of coal in oxygen given by the stoichiometric equation: CHN 0:01 O 0:1 S 0:05 + aO 2 = bCO 2 + cH 2 O + dN 2 + eSO 2
-
Determine the value of the stoichiometric coefficients for the combustion of natural gas in air: CH 4 + a(O 2 + 3:76N 2 ) = bCO 2 + cH 2 O + dN 2
-
Using the stoichiometric coefficients you found in exercise 9, determine the molar air-to-fuel ratio (A/F) molar for the combustion of natural gas in air. (A: (A/F) molar = 9.52 kmol of air/kmol of...
-
On NSE (Indian stock exchange), shares of ICICI Bank trade for 935 rupees. If the spot exchange rate is USD 0.012, what is the no-arbitrage USD price of ICICI Bank ADR? Assume that transactions costs...
-
Income Statement Balance Sheet Balance Sheet Additional Financial Information 1. Market price of Ranfield's common stock: $90.44 at December 31, 2024, and $58.35 at December 31, 2023. 2. Common...
-
There is a credit rating agency for businesses that gives out various amounts of information based on the subscription level. This company is called a. Business Credit Scoring b. Fair Issue c. Dun...

Study smarter with the SolutionInn App


