A 17.3-liter tank contains a mixture of argon, helium, and nitrogen at 298 K. The argon and
Question:
A 17.3-liter tank contains a mixture of argon, helium, and nitrogen at 298 K. The argon and helium mole fractions are 0.12 and 0.35, respectively. If the partial pressure of the nitrogen is 0.8 atm, determine
(a) The total pressure in the tank,
(b) The total number of moles (kmol) in the tank,
(c) The mass of the mixture in the tank.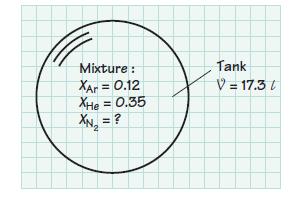
Transcribed Image Text:
Mixture : XAr = 0.12 XHe = 0.35 Tank V = 17.3 /
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (8 reviews)
To solve this problem we will use the ideal gas law which states PV nRT where P is the pressure V is the volume n is the number of moles R is the gas ...View the full answer

Answered By

Isabel Seraspi
I have experience teaching math, science, and English to students of all ages. I have also worked as a tutor in a college setting, helping students with their homework and preparing them for exams.
I believe that tutoring is a great way to help students learn. It allows students to get one-on-one help with their studies, and it gives them the chance to ask questions and get immediate feedback. Tutoring can also be tailored to the individual needs of the student, which is why I believe it is so effective.
I have seen firsthand how tutoring can help students improve their grades and confidence. I have also seen how it can help students who are struggling with a particular subject. I believe that tutoring is a great way to help students learn and succeed in school.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Thermodynamics Concepts And Applications
ISBN: 9781107179714
2nd Edition
Authors: Stephen R. Turns, Laura L. Pauley
Question Posted:
Students also viewed these Engineering questions
-
1 723 Conditions for promotions 5 Years of service (Years) 6 Psychometric test (%) Required: a) LIST OF EMPLOYEES FOR PROMOTION 9 Names of employees Years of service 10 Munawarah Ali 11 Amiruddin...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
At 298 K the standard enthalpy of combustion of sucrose is -5797 k] mol-I and the standard Gibbs energy of the reaction is -6333 k] mol ". Estimate the additional non-expansion work that may be...
-
You work for a gas turbine design company and have a client who has a fairly loose specification for a gas turbine engine. You are required to design an aviation gas turbine to power the aircraft...
-
At the end of 2012, Tootsie Roll had a price- earnings ratio of 30.7, and Hershey had a price- earnings ratio of 25.3. These convert to capitalization rates of 3.25% for Tootsie Roll and 3.95% for...
-
6. LO.7 Rosalie owns 50% of the outstanding stock of Salmon Corporation. In a qualifying stock redemption, Salmon distributes $80,000 to Rosalie in exchange for one-half of her shares, which have a...
-
Debido a que provengo de un entorno de recompensas y lealtad, a menudo me pregunto cmo combinar las mejores recompensas y comisiones con el universo en expansin de las redes sociales, dice Jennifer...
-
Bridgeport Delivery Service is owned and operated by Jerome Foley. The following selected transactions were completed by Bridgeport Delivery Service during February: 1. Received cash from owner as...
-
Suppose that the annual volatility of market returns is 10%. For how many years would you need to calculate a sample mean return in order that the standard error of your estimate is 0.1%? (Nearest 1...
-
For each of the following items, indicate how the financial statements will be affected. Identify the affected accounts specifically. 1. Whirlpool Corporation sells electric appliances, including...
-
Determine the total apparent specific heat at constant pressure (c p,mix in kJ/kg K) for a fuelair reactant mixture containing 1 kmol CH 4 , 2.5 kmol O 2 , and 9.4 kmol N 2 at 500 K and 1 atm. Use...
-
In Table C.2, at what reference temperature and pressure is the entropy zero? TABLE C.2 Thermodynamic Properties of Air at 1 atm* h (kJ/kg) u (kJ/kg) s (kJ/kg-K) 325.42 268.14 3.4764 335.49 275.32...
-
Mutt and Jeff are discussing how income and losses should be divided in a partnership they plan to form. What factors should be considered in determining the division of net income or net loss?
-
speed of the three phase motor does not vary greatly from the experiment. 1. Draw the symbol for a Three Phase Electric Motor. (Hint: remember the symbol table from the beginning of the semester?) 2....
-
Lifetime Insurance Company has two supporting departments (actuarial and premium), and two production departments (advertising and sales). Data from operations for the current year are as follows:...
-
Consider a wireless local area network (LAN) with an access point and 10 stations (Station 1, Station 2, Station 3, , and Station 10). Distributed coordination function (DCF), which is based on...
-
A worker needs to pump water from a reservoir to a big container that is open to the atmosphere. The water velocity at the surface of the reservoir is 2.5 m/s. The worker uses a 35-m long, 18-cm...
-
Identify each fringe benefit provided to Maggie and determine whether an exemption applies. (6 marks) Question 2: Explain the impact the fringe benefits will have on Maggie's taxable income and/or...
-
Propose a plausible mechanism for the following transformation. NaOH, H20 Heat
-
One study found that the elderly who do not have children dissave at about the same rate as the elderly who do have children. What might this finding imply about the reason the elderly do not dissave...
-
Using the results of exercises 9 and 10, determine the mass air-to-fuel ratio (A/F) mass for the combustion of natural gas in air. (A: 17.2 kg of air/kg of fuel)
-
Determine the mass air-to-fuel ratio (A/F) mass for the combustion of an oil (represented by CH 2 ) in air.
-
Determine the mass air-to-fuel ratio (A/F)mass for the combustion of coal in air represented by the equation: CHN 0:01 O 0:1 S 0:05 + a(O 2 + 3:76N 2 ) = bCO 2 + cH 2 O + dN 2 + eSO 2
-
Current Attempt in Progress On July 3 1 , 2 0 2 2 , Crane Compary had a cash balance per books of $ 6 , 2 4 5 . 0 0 . The statement from Dakata State Bark on that date showed a balance of $ 7 , 7 9 5...
-
Cede & Co. expects its EBIT to be $89,000 every year forever. The firm can borrow at 5 percent. Cede currently has no debt, and its cost of equity is 10 percent. If the tax rate is 35 percent, what...
-
In the Marriott example, one discussion point considered when a firm might use a single hurtle rather than different divisional or business unit rates. When a single rate is used and the divisions...

Study smarter with the SolutionInn App


