Question: A laboratory analysis shows that CO 2 becomes 10 % dissociated into CO and O 2 at 2390 K if the total pressure is 1
A laboratory analysis shows that CO2 becomes 10 % dissociated into CO and O2 at 2390 K if the total pressure is 1 atm. Obtain the equilibrium constant from this information and compare it to the value given in Table D.1.
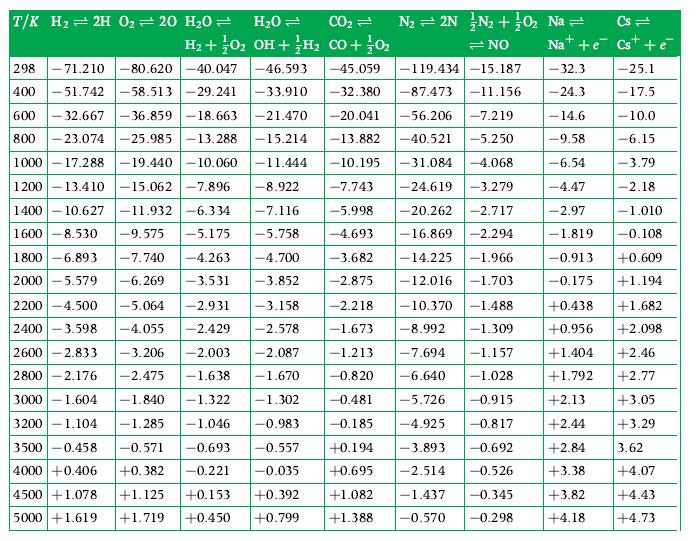
T/K H2H 0 = 20 H0 = HO = CO = N = 2N N + 0 Na = Cs= H + O2 OH + H CO + 0 =NO Nat+e Cs + -45.059 -32.3 -32.380 -87.473 -11.156 -24.3 -20.041 -56.206 -7.219 - 14.6 -13.882 -40.521 -5.250 -9.58 -10.195 -31.084 -4.068 -6.54 298 -71.210 -80.620-40.047 -46.593 400 -51.742-58.513 -29.241 -33.910 600 -32.667 -36.859-18.663 -21.470 800-23.074 -25.985 -13.288 -15.214 1000-17.288 -19.440 -10.060 -11.444 1200-13.410 -15.062 -7.896 -8.922 1400-10.627 -11.932-6.334 -7.116 1600 -8.530 -9.575 -5.175 -5.758 1800 -6.893 2000 -5.579 -4.47 -2.97 -7.743 -24.619 -3.279 -5.998 -20.262 -2.717 -4.693 -16.869 -2.294 -3.682 -14.225 -1.966 -2.875 -12.016 -1.703 -1.819 -7.740 -4.263 -4.700 -3.852 -6.269 -3.531 2200 -4.500 -5.064 -2.931 2400 -3.598 -4.055 -2.429 2600 -2.833 -3.206 -2.003 2800 -2.176 -2.475 -1.638 3000 -1.604 -1.840 -1.322 -2.218 -10.370 -1.488 -1.673 -8.992 -1.309 -7.694 -1.157 -6.640 -1.028 -5.726 -0.915 -4.925 -0.817 -3.893 -0.692 -2.514 -0.526 -0.345 -0.298 -3.158 -2.578 -2.087 -1.213 -1.670 -0.820 - 1.302 -0.481 -0.185 +0.194 +0.695 3200-1.104 -1.285 -1.046 -0.983 3500 -0.458 -0.571 -0.693 -0.557 4000 +0.406 +0.382 -0.221 -0.035 4500 +1.078 +1.125 +0.153 5000 +1.619 +1.719 +0.450 +0.392 +0.799 -119.434-15.187 +1.082 -1.437 +1.388. -0.570 -25.1 -17.5 -10.0 -6.15 -3.79 -2.18 -1.010 -0.108 -0.913 +0.609 -0.175 +1.194 +1.682 +2.098 +2.13 +2.44 +2.84 +3.38 +0.438 +0.956 +1.404 +2.46 +1.792 +2.77 +3.05 +3.29 3.62 +4.07 +3.82 +4.43 +4.18 +4.73
Step by Step Solution
3.33 Rating (162 Votes )
There are 3 Steps involved in it
The reaction can be written as CO CO 050 As the laboratory analysi... View full answer

Get step-by-step solutions from verified subject matter experts


