The oxidation of methane can take place according to either one of the following reactions: When the
Question:
The oxidation of methane can take place according to either one of the following reactions: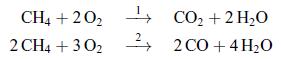
When the reactions stop at time tf because all the methane is burned, the total mass of the products (CO2, CO, H2O) is![]() Determine the initial mass of methane mCH4 (0) in terms of the total mass of the products m(tf) and the mass of water mH2O (tf). Numerical Application:
Determine the initial mass of methane mCH4 (0) in terms of the total mass of the products m(tf) and the mass of water mH2O (tf). Numerical Application:![]()
Transcribed Image Text:
CH4 +20₂ CO₂ + 2H₂O 2 CH4 +30₂22CO+ 4H₂O
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 44% (9 reviews)
We need to find the initial mass of methane mCH40 in terms of the total mass of the products mtf and ...View the full answer

Answered By

Alex Maina Muigai
I am a recent graduate of Maseno University, where I earned a Bachelor of Science degree in Chemical Engineering. During my studies, I developed a strong knowledge base in chemical engineering principles, including chemical reaction engineering, fluid mechanics, and thermodynamics. I also gained extensive experience in laboratory techniques and process engineering.
Since graduating, I have been working as a tutor in the field of chemical engineering. I have been providing guidance and support to students in the topics of chemical engineering, including designing chemical processes, designing experiments, and troubleshooting problems. I am also proficient in mathematics, physics, and chemistry, which I use to help my students understand the concepts of chemical engineering.
I have a passion for teaching and helping others understand complex concepts. I have a strong commitment to always providing accurate and reliable information to my students, and I strive to ensure that they have a positive learning experience. I am confident that my knowledge and experience in chemical engineering will be an asset to any student who chooses to work with me.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Principles Of Thermodynamics
ISBN: 9781108426091
1st Edition
Authors: Jean-Philippe Ansermet, Sylvain D. Brechet
Question Posted:
Students also viewed these Engineering questions
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
When a pure substance is placed in contact with water, there are three possible outcomes. The substance may do nothing that is, the substance does not dissolve and no visible change takes place. The...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
Smart Sets manufactures headphone cases. During September 2016, the company produced 108,000 cases and recorded the following cost data: Requirements 1. Compute the cost and efficiency variances for...
-
Spencer Cook died on July 18 of the current year, leaving a gross estate of $4,600,000. Claims to be settled against that estate included funeral, administrative, and medical expenses of $180,000 and...
-
Is it appropriate for baseball managers to use only quantitative, objective criteria in evaluating their players? What do you think? Why?
-
Many companies use well-known celebrities in their ads, while other companies create their own spokespersons (such as the Maytag repairman). A study in the Journal of Marketing (Fall 1992)...
-
Willard Motors, Inc., employs 20 sales personnel to market its line of luxury automobiles. The average car sells for $65,000, and a 6 percent commission is paid to the salesperson. Willard Motors is...
-
h of the following statements is correct regarding the relationship of ending inventory and begini nding inventory of the previous period is the beginning inventory of the current period. eginning...
-
Repeat Example 9.2, but with a feed pressure of 5.0 MPa. Compare your results to those obtained at a feed pressure of 1.0 \(\mathrm{MPa}\). Data From Example 9.2:- Air can be separated into...
-
Apply the general definition of the battery potential, to the Daniell cell ( 8.7.4) and show that it yields relation (8.108). Show that the battery potential can be written as, where - 1 zFF A VaA PA
-
Acetylene (C 2 H 2 ) can be produced through a chemical reaction between water (H 2 O) and calcium carbide (C a C 2 ): where (s) and (l) indicate whether the substance is solid or liquid. A cave...
-
If you suspected skimming of sales at the cash register, what is one of the first things you would check?
-
Provide a brief bio of the leader and a brief overview of the change or crisis they led the organization or movement through. Discuss their leadership style during this change/crisis using one of the...
-
Write a C++ function named Ifsr that accepts feedback path and initial states as unsigned integers and the number of random bits to be printed as arguments. The function will print the random bits by...
-
Newfoundland Hapset will be remitted to _____
-
The Z Energy Corp. has a new investment opportunity that generates cash flows of $6 million per year (in expectation) forever. The managers of Z Energy are not sure what the required rate of return...
-
Define an interface TwoStrings Oper declaring a function apply which takes two strings and returns a string. Then, define four classes implementing this interface, where the operation on strings...
-
Refer to the previous exercise. Instead of 49, suppose that 64 Americans were surveyed about their weekly expenditures on coffee. Assume the sample mean remained the same. a. What is the 95%...
-
Cable Corporation is 60% owned by Anna and 40% owned by Jim, who are unrelated. It has noncash assets, which it sells to an unrelated purchaser for $100,000 in cash and $900,000 in installment...
-
Obtain the expression for the isothermal compressibility from the Helmholtz energy a = a - (T,v).
-
How can the residual part of c P of a simple compressible fluid be evaluated from an an equation of state in the form P = P - (T, v)?
-
Work out the differential relation between specific entropy of a simple compressible fluid and its temperature and pressure, such that it can be evaluated with the help of c P and an equation of...
-
If Total Assets are $2,100 and Total Equity is $1,600 then what is the value of Total Liabilities?
-
Date Account and Explanation Debit Credit Jun 1 Cash 105,000 Jun 1 Capital 105,000 (capital contribution) Jun 1 Computer Equipment 56,000 Jun 1 Cash 56,000 Jun 1 Cash 198,000 Jun 1 Bank Loan Payable...
-
.Is bankruptcy on the part of the borrower a common risk that frequently interferes with a lenders efforts to work out a defaulted loan through either nonforeclosure means or foreclosure? Discuss.

Study smarter with the SolutionInn App


