Question: The water-gas shift reaction is ?Processes based on this reaction have been studied as a means of providing H 2 as an automotive fuel. Assume
The water-gas shift reaction is ![]() ?Processes based on this reaction have been studied as a means of providing H2 as an automotive fuel. Assume that 1 mole of CO reacts with one mole of H2O at1800 K and 1 atm. Determine the equilibrium composition. Will lowering the temperature increase or decrease the yield of H2? Neglect species other than CO, H2O, CO2 , H2. Values of K = K( - T) for the water-gas shift reaction are
?Processes based on this reaction have been studied as a means of providing H2 as an automotive fuel. Assume that 1 mole of CO reacts with one mole of H2O at1800 K and 1 atm. Determine the equilibrium composition. Will lowering the temperature increase or decrease the yield of H2? Neglect species other than CO, H2O, CO2 , H2. Values of K = K( - T) for the water-gas shift reaction are
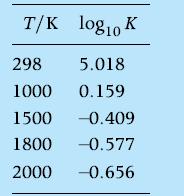
What effect does an increase in CO have on the H2?yield at 1800 K?
CO+H,O = CO,+H,.
Step by Step Solution
3.32 Rating (158 Votes )
There are 3 Steps involved in it
The watergas shift reaction is COH0 COH The equilibrium constant ... View full answer

Get step-by-step solutions from verified subject matter experts


