Calculate the equilibrium composition of the water-gas shift reaction of Exercise 10.25 with the element potential method.
Question:
Calculate the equilibrium composition of the water-gas shift reaction of Exercise 10.25 with the element potential method. Compare the accuracy of the results with those obtained with the equilibrium constant method and with STANJAN, commenting on the difference and on the differences between the methods. Make a chart of the equilibrium composition as a function of temperature similar to the one of Figure 10.5a. Comment on the trends visible in the chart.
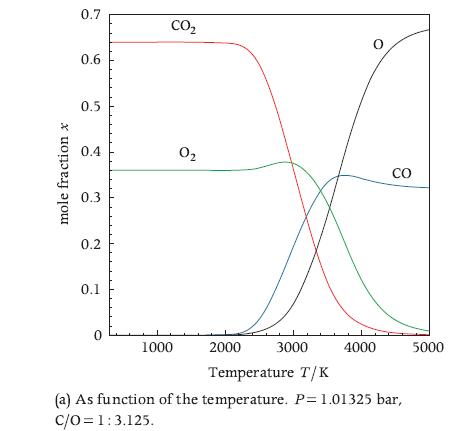
Data From Exercise 10.25
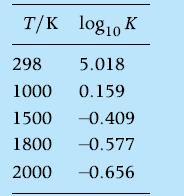
The water-gas shift reaction is ![]() . Processes based on this reaction have been studied as a means of providing H2 as an automotive fuel. Assume that 1 mole of CO reacts with one mole of H2O at1800 K and 1 atm. Determine the equilibrium composition. Will lowering the temperature increase or decrease the yield of H2? Neglect species other than CO, H2O, CO2 , H2. Values of K = K( - T) for the water-gas shift reaction are
. Processes based on this reaction have been studied as a means of providing H2 as an automotive fuel. Assume that 1 mole of CO reacts with one mole of H2O at1800 K and 1 atm. Determine the equilibrium composition. Will lowering the temperature increase or decrease the yield of H2? Neglect species other than CO, H2O, CO2 , H2. Values of K = K( - T) for the water-gas shift reaction are
What effect does an increase in CO have on the H2?yield at 1800 K?
Step by Step Answer:

Thermodynamics Fundamentals And Engineering Applications
ISBN: 9780521862738
1st Edition
Authors: William C. Reynolds, Piero Colonna





