To establish the positivity of the specific heat at constant pressure Cp compressibility coefficient at constant temperature
Question:
To establish the positivity of the specific heat at constant pressure Cp compressibility coefficient at constant temperature κT (see relations (6.31)), follow the steps given here [65]:a) Show that the Mayer relation (5.42) can be recast as,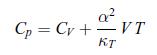 where α is the thermal coefficient of expansion,
where α is the thermal coefficient of expansion,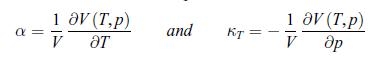
b) Show that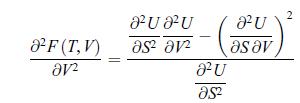
c) Conclude from these two results that κT ≥ 0 and Cp ≥ 0.
Transcribed Image Text:
Cp=Cv+VT КТ
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (12 reviews)
To establish the positivity of the specific heat at constant pressure Cp and the compressibility coe...View the full answer

Answered By

Ragul r
I am highly experienced and dedicated educator with a passion for teaching students of all ages. With over
4 years of experience in education and tutoring, I have helped countless students achieve their academic goals in subjects such as math and science.
My journey in education began with a Bachelor's degree in from Maseno University, where I developed a strong foundation in critical thinking and problem-solving skills that have proved invaluable in my teaching career.
I am committed in helping your students in IT and various topics in sciences and math
0.00
0 Reviews
10+ Question Solved
Related Book For 

Principles Of Thermodynamics
ISBN: 9781108426091
1st Edition
Authors: Jean-Philippe Ansermet, Sylvain D. Brechet
Question Posted:
Students also viewed these Engineering questions
-
The specific heat at constant pressure for an ideal gas is given by cp = 0.9 + (2.7 x 10-4) T (kJ/kg K) where T is in kelvin. The change in the enthalpy for this ideal gas undergoing a process in...
-
The specific enthalpy (h) of an ideal gas is a function of temperature (T) only as can be seen from Table D-3 for air. (a) Using the data from the table, determine the specific heat at constant...
-
The specific heat at constant pressure cp [J/(kg K)] of an ideal gas is related to enthalpy by where h = enthalpy (kJ/kg), and T = absolute temperature (K). The following enthalpies are provided for...
-
Question 4: Artistic Hand for engineering Services was formed on Jun 1, 2018. The following transactions took place during the month of Jun: I The owner invested $20,000 cash in business. 2 Hired an...
-
The following events occurred as part of the operations of Craig State University, a public university: 1. To construct a new computer complex, the university floated at par a $22,000,000, 7% serial...
-
10.2 Priestley and Judge are partners, sharing profits and losses in the ratio 3 : 2. The following is the trial balance in the partnership books at 31 December 20X6. Capital account at 1.1.X6:...
-
A multinomial experiment with k = 4 cells and n = 205 produced the data shown in the one-way table below. a. Do these data provide sufficient evidence to conclude that the multinomial probabilities...
-
The cash records of Dawes Company show the following four situations. 1. The June 30 bank reconciliation indicated that deposits in transit total $920. During July, the general ledger account Cash...
-
ABC irketi'nin muhasebe kaytlar 31 Aralk 2022 yl sonu itibaryla aadaki gibidir. Satn Alma ndirimleri | 3.400 $ | Navlun | 6.100 $ ---|---|---|--- Satn Almalar | 162.500 | Balang Stoklar | 18.000 Biti...
-
An investor, wants to know how two portfolios are performing in the market: 7% Y (3%) 12% Probability Market portfolio 0.40 0.20 15% 0.40 Beta factor 10% 18% Portfolio X 1.30 10% 8% 4% 0.75 1 The...
-
An isolated system consisting of two closed subsystems A and B is separated by a diathermal wall. Initially, they are held at temperatures TiA and TiB . Subsystem A contains NA moles of gas. The...
-
A system consists of a thin film of surface area A, of internal energy U(S, A), where Hence, the surface tension is given by Express the heat Qif to provide to the film for a variationAif = AfAi of...
-
A small body A starts sliding off the top of a smooth sphere of radius R Find the angle θ (Fig. 125) corresponding to the point at which the body breaks off the sphere, as well as the break-off...
-
Suppose a company bases its hourly rates on the number of customers per hour. The hourly rate the company charges is given by two functions where = g(2) 4, g(3) = 2, 9(4) = 3 and f(2) = 6, f(3) = 3,...
-
Which statements about insurance are true? 1- Insurance protects against the the worst-case scenario. All rational people want to buy insurance. 2- Insurance costs money, and therefore always...
-
need step by step instruction about creating this: in NX12 PART NAME: BRACKET ALL FILLETS R .313 ALL ROUNDS R .625 2X .500 1/500 2.875 9.500 4750 2875 $500 3.000 750 GENTERED IN OBJECT 2.375
-
8. Convert the angle - 7t from radian measure into degree measure. Show some work. 4
-
4. Variance Analysis. (CPA, adapted) The H. G. Company uses a standard cost system in accounting for the cost of one of its products. < The Budget is based on normal capacity of monthly production of...
-
A recent national survey found that parents read an average (mean) of 10 books per month to their children under five years old. The population standard deviation is 5. The distribution of books read...
-
What is taxable income, and what is the formula for determining taxable income?
-
In an adiabatic constant-volume container, a fluid is to be stirred mechanically with a motor providing 40 W of shaft power. How long can this go on if the internal energy of the fluid is not allowed...
-
Calculate the density of gaseous H 2 O at each of the following states:a. 0.07 bar, 360 K,b. 1 atm, 700 K,c. 200 bar, 700 K,Using the perfect gas model. Compare the values obtained with the values...
-
The temperature of 10 kg of air inside a pistoncylinder is increased from 25C to 80C using an electrical heater, while the pressure inside the cylinder is maintained constant. During this process 50...
-
Duncan Inc. issued 500, $1,200, 8%, 25 year bonds on January 1, 2020, at 102. Interest is payable on January 1. Duncan uses straight-line amortization for bond discounts or premiums. INSTRUCTIONS:...
-
WISE-HOLLAND CORPORATION On June 15, 2013, Marianne Wise and Dory Holland came to your office for an initial meeting. The primary purpose of the meeting was to discuss Wise-Holland Corporation's tax...
-
Stock in ABC has a beta of 0.9. The market risk premium is 8%, and T-bills are currently yielding 5%. The company's most recent dividend is $1.60 per share, and dividends are expected to grow at a 6%...

Study smarter with the SolutionInn App


