Question: Your company asks you, the process engineer, to estimate the rate at which energy is transferred as heat through a steam turbine casing. This turbine
Your company asks you, the process engineer, to estimate the rate at which energy is transferred as heat through a steam turbine casing. This turbine operates with an inlet pressure of 16 bar and an inlet temperature of 377°C, while the steam exits the turbine as saturated vapor at 30°C. Its shaft power is 9 MW. The mass flow rate of steam is 15 kg/s. Use Appendix A.3 for data.
Data From A.3
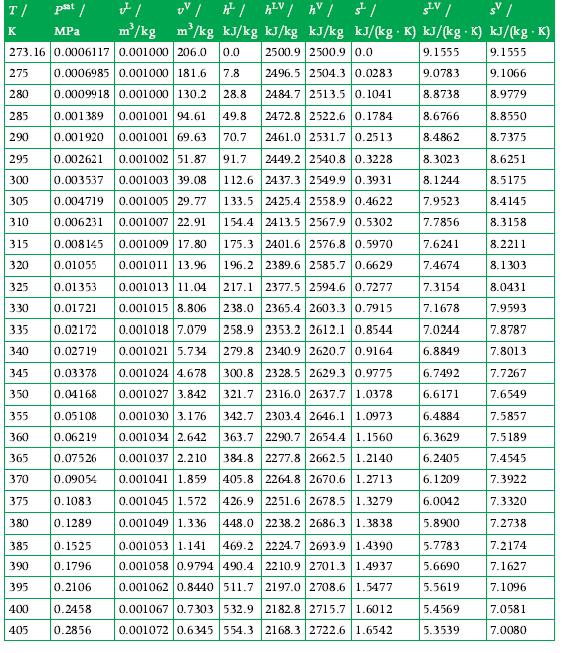
T/ psat / K MPa UV /h/hV / hv / st/ SLV / s | m/kg kJ/kg kJ/kg kJ/kg kJ/(kg. K) kJ/(kg K) kJ/(kg .K) 2500.9 2500.9 0.0 9.1555 9.1555 2496.5 2504.3 0.0283 9.0783 9.1066 2484.7 2513.5 0.1041 8.8738 8.9779 FI m/kg 273.16 0.0006117 0.001000 206.0 0.0 275 0.0006985 0.001000 181.6 7.8 0.0009918 0.001000 130.2 28.8 280 285 0.001389 0.001001 94.61 49.8 2472.8 2522.6 0.1784 290 0.001920 0.001001 69.63 70.7 2461.0 2531.7 0.2513 295 0.002621 0.001002 51.87 91.7 2449.2 2540.8 0.3228 300 0.003537 0.001003 39.08 112.6 2437.3 2549.9 0.3931 133.5 2425.4 2558.9 0.4622 305 0.001005 29.77 0.004719 0.006231 0.001007 22.91 154.4 2413.5 2567.9 0.5302 310 175.3 2401.6 2576.8 0.5970 196.2 2389.6 2585.7 0.6629 217.1 2377.5 2594.6 0.7277 238.0 2365.4 2603.3 0.7915 258.9 2353.2 2612.1 0.8544 279.8 2340.9 2620.7 0.9164 315 0.008145 0.001009 17.80 320 0.01055 0.001011 13.96 0.01353 0.001013 11.04 0.01721 0.001015 8.806 335 0.02172 325 330 0.001018 7.079 0.02719 0.001021 5.734 340 345 0.03378 350 0.04168 355 0.05108 360 0.06219 365 0.07526 370 0.09054 375 0.1083 380 0.1289 385 0.1525 390 0.1796 395 0.2106 0.2458 400 405 0.2856 0.001024 4.678 300.8 2328.5 2629.3 0.9775 321.7 2316.0 2637.7 1.0378 0.001027 3.842 0.001030 3.176 2303.4 2646.1 1.0973 342.7 363.7 2290.7 2654.4 1.1560 0.001034 2.642 0.001037 2.210 0.001041 1.859 384.8 2277.8 2662.5 1.2140 405.8 2264.8 2670.6 1.2713 426.9 0.001045 1.572 2251.6 2678.5 1.3279 448.0 2238.2 2686.3 1.3838 0.001049 1.336 0.001053 1.141 469.2 2224.7 2693.9 1.4390 0.001058 0.9794 490.4 2210.9 2701.3 1.4937 0.001062 0.8440 511.7 2197.0 2708.6 1.5477 2182.8 2715.7 1.6012 0.001067 0.7303 532.9 0.001072 0.6345 554.3 2168.3 2722.6 1.6542 8.6766 8.4862 8.3023 8.1244 7.9523 7.7856 7.6241 7.4674 7.3154 7.1678 7.0244 6.8849 6.7492 6.6171 6.4884 6.3629 6.2405 6.1209 6.0042 5.8900 5.7783 5.6690 5.5619 5.4569 5.3539 8.8550 8.7375 8.6251 8.5175 8.4145 8.3158 8.2211 8.1303 8.0431 7.9593 7.8787 7.8013 7.7267 7.6549 7.5857 7.5189 7.4545 7.3922 7.3320 7.2738 7.2174 7.1627 7.1096 7.0581 7.0080
Step by Step Solution
3.33 Rating (156 Votes )
There are 3 Steps involved in it
1 Make an idealized sketch of the system and identify the boundary indicating if it moves in time 2 ... View full answer

Get step-by-step solutions from verified subject matter experts


