In Fig. E24.20, C 1 = 3.00 µF and V ab = 150 V. The charge on
Question:
Fig.24.20
Transcribed Image Text:
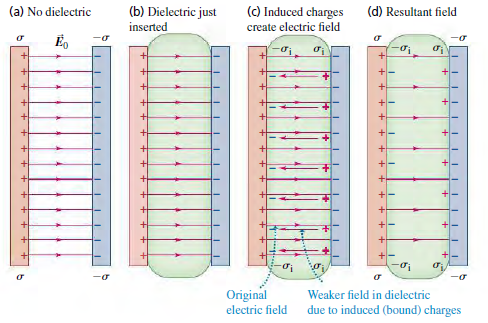
(C) Induced charges create electric field (a) No dielectric (b) Dielectric just (d) Resultant field inserted Eo Original electric field Weaker field in dielectric due to induced (bound) charges
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 35% (14 reviews)
IDENTIFY Capacitors in series carry the same charge while capacitors in parallel have the same p...View the full answer

Answered By

GERALD KAMAU
non-plagiarism work, timely work and A++ work
4.40+
6+ Reviews
11+ Question Solved
Related Book For 

University Physics with Modern Physics
ISBN: 978-0133977981
14th edition
Authors: Hugh D. Young, Roger A. Freedman
Question Posted:
Students also viewed these Physics questions
-
In Figure a potential difference of V = 100.0 V is applied across a capacitor arrangement with capacitances C1 = 10.0F, C2 = 5.00F, and C3 = 4.00F, If capacitor 3 undergoes electrical breakdown so...
-
In Figure the capacitances arc C1 = 1.0F and C2 = 3.0F, and both capacitors are charged to a potential difference of V = 100 V but with opposite polarity as shown. Switches S1 and S2 are now closed....
-
In Figure V = 9.0 V, C1 = C2 = 30F, and C3 = C4 = 15F. What is the charge on capacitor 4? C2
-
In Problems 530, a. Classify the sequences as arithmetic, geometric, Fibonacci, or none of these. b. If arithmetic, give d; if geometric, give r; if Fibonacci, give the first two terms; and if none...
-
The pressure in an ideal gas is cut in half slowly, while being kept in a container with rigid walls. In the process, 465 kJ of heat left the gas. (a) How much work was done during this process? (b)...
-
Along with the Report of Condition submitted (question 5.1 of text), Norfolk has also prepared a Report of Income for the FDIC. Please fill in the missing items from its statement shown below (all...
-
Give an example of how a rule could fit the corporate strategy of cost leadership. LO.1
-
The 12 partners of a regional public accounting firm met in special session to discuss audit engagement efficiency. Jones spoke up, saying, We all certainly appreciate the firm wide policies set up...
-
Required information Use the following information for the Problems below. (Algo) [The following information applies to the questions displayed below.) Lansing Company's current-year income statement...
-
Kara Shin is a licensed CPA. During the first month of operations of her business, Kara Shin, Inc., the following events and transactions occurred. May 1 Stockholders invested $20,000 cash in...
-
Polystyrene has dielectric constant 2.6 and dielectric strength 2.0 10 7 V/m. A piece of polystyrene is used as a dielectric in a parallel-plate capacitor, filling the volume between the plates. (a)...
-
A potential difference V ab = 48.0 V is applied across the capacitor network of Fig. E24.17. If C 1 = C 2 = 4.00 µF and C 4 = 8.00 µF, what must the capacitance C 3 be if the network is...
-
A 0.010 M solution of ammonia, NH3, has a pH of 10.6 at 25C. What is the concentration of hydroxide ion?
-
Use least square regression to fit a straight line to the following data taken from the conductance (S/m) of a material with respect to temperature (C) of a composite material used to absorb heat....
-
A pile group consists of nine friction piles in clay soil (see Figure 10-40). The diameter of each pile is 16 in., and the embedded length is 30 ft each. Center-to-center pile spacing is 4 ft. Soil...
-
The rigid bar EBC is supported by two links AB and CD as shown in Figure 1. The Link AB is made of aluminum (E = 70 GPa) and the link CD is made of steel (E = 200 GPa). Both links have a Width = 30...
-
a well-insulated storage tank was pressurized under ideal gas conditions by air flowing into the tank. We used the first law to estimate the final temperature of the gas in the tank, Tf,tank- = We...
-
Transportation of natural gas is commonly done via pipelinesacross long distances. A company uses a 0.6-m diameter pipe totransport natural gas. Then pumping stations are located atdifferent points...
-
A helicopter is rising straight up in the air. Its distance from the ground t seconds after takeoff is s(t) feet, where s(t) = t 2 + t. (a) How long will it take for the helicopter to rise 20 feet?...
-
Question 2 For an n x n matrix A = form) via (aij)
-
Helium gas with a volume of 2.60 L, under a pressure of 0.180 atm and at a temperature of 41.0 o C, is warmed until both pressure and volume are doubled. a. What is the final temperature? b. How many...
-
A cylindrical tank has a tight-fitting piston that allows the volume of the tank to be changed. The tank originally contains 0.110 m 3 of air at a pressure of 0.355 atm. The piston is slowly pulled...
-
(a) Calculate the density of the atmosphere at the surface of Mars (where the pressure is 650 Pa and the temperature is typically 253 K, with a CO 2 atmosphere), Venus (with an average temperature of...
-
3. How much life insurance do you need? Calculating resources - Part 2 Aa Aa E Paolo and Maria Rossi have completed Step 1 of their needs analysis worksheet and determined that they need $2,323,000...
-
On March 1, LGE asks to extend its past-due $1,200 account payable to Tyson, Tyson agrees to accept $200 cash and a 180-day, 8%, $1,000 note payable to replace the account payable. (Use 360 days a...
-
*Prepare the plant assets section of Amphonie's balance sheet at December 31, 2021 using the information below. At December 31, 2020, Amphonie Company reported the following as plant assets. Land $...

Study smarter with the SolutionInn App


