Question: The electron in a hydrogen atom can be considered to be in a circular orbit with a radius of 0.0529 nm and a kinetic energy
The electron in a hydrogen atom can be considered to be in a circular orbit with a radius of 0.0529 nm and a kinetic energy of 13.6 eV. If the electron behaved classically, how much energy would it radiate per second (see Challenge Problem 32.57)? What does this tell you about the use of classical physics in describing the atom?
Problem 32.57
Electromagnetic radiation is emitted by accelerating charges. The rate at which energy is emitted from an accelerating charge that has charge q and acceleration a is given by
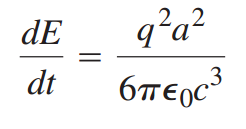
where c is the speed of light.
qa? dE dt 603
Step by Step Solution
3.45 Rating (165 Votes )
There are 3 Steps involved in it
Identify The electron has acceleration a v 2 R Set ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (2 attachments)
1477_605aea4cb06a8_682282.pdf
180 KBs PDF File
1477_605aea4cb06a8_682282.docx
120 KBs Word File


