Question: Use Table 41.3 to help determine the ground-state electron configuration of the neutral gallium atom (Ga) as well as the ions Ga+ and Ga-. Gallium
Use Table 41.3 to help determine the ground-state electron configuration of the neutral gallium atom (Ga) as well as the ions Ga+ and Ga-. Gallium has an atomic number of 31.
Table 41.3
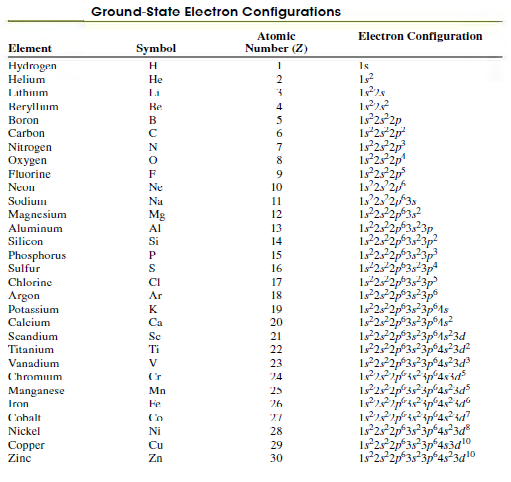
Ground-State Electron Configurations Atomic Electron Configuration Element Symbol Number (Z) Hydmgen Helium H Is Lithium Reryllum Boron Re 4 Is252p 1s252p 1s 25 2p 125 2p 1s 2 2p Carbon 6. Nitrogen en 7 8 Fluorine 9. Neun Ne 10 Sudium Na 11 1522p3,? 12,2323p 122 Magnesium Mg 12 Aluminum 13 Silicon Si 14 Phosphorus Sulfur P 15 S 16 1s22p*3s*3p 1s22p3s3p Chlorine CI 17 Argon Ar 18 Potassium K 19 1s252p3s3p1s 1s252p3s3p1s3d Calcium Ca 20 Scandium Sc 21 Titanium Ti 22 122p3s3p4s3d Vanadium V 23 Chromium 24 Manganese Iron Mn 25 Fe 26 Cobalt Nickel Ni 28 Copper Zinc Cu 29 Zn 30
Step by Step Solution
3.43 Rating (156 Votes )
There are 3 Steps involved in it
Zinc Z 30 has filled subshells through 4s 2 3d 10 The nex... View full answer

Get step-by-step solutions from verified subject matter experts


