Suggest a structure for the compound whose mass spectrum is asfollows: 100 107 81 93 188 70
Question:
Suggest a structure for the compound whose mass spectrum is asfollows:
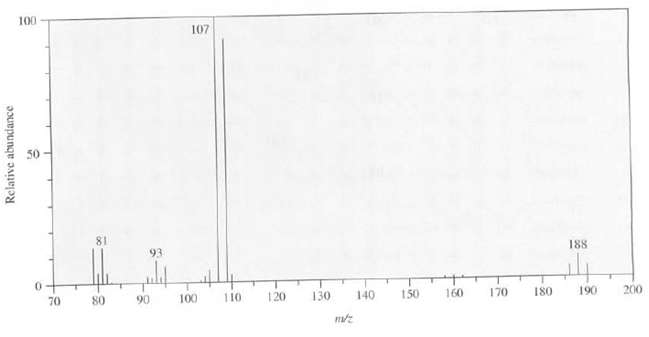
Transcribed Image Text:
100 107 81 93 188 70 80 110 120 90 100 130 150 160 170 140 180 190 200 Relative abundance
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
The peaks at mz 186 188 and 190 in a 121 ratio suggests the pres...View the full answer

Answered By

Asim farooq
I have done MS finance and expertise in the field of Accounting, finance, cost accounting, security analysis and portfolio management and management, MS office is at my fingertips, I want my client to take advantage of my practical knowledge. I have been mentoring my client on a freelancer website from last two years, Currently I am working in Telecom company as a financial analyst and before that working as an accountant with Pepsi for one year. I also join a nonprofit organization as a finance assistant to my job duties are making payment to client after tax calculation, I have started my professional career from teaching I was teaching to a master's level student for two years in the evening.
My Expert Service
Financial accounting, Financial management, Cost accounting, Human resource management, Business communication and report writing. Financial accounting : • Journal entries • Financial statements including balance sheet, Profit & Loss account, Cash flow statement • Adjustment entries • Ratio analysis • Accounting concepts • Single entry accounting • Double entry accounting • Bills of exchange • Bank reconciliation statements Cost accounting : • Budgeting • Job order costing • Process costing • Cost of goods sold Financial management : • Capital budgeting • Net Present Value (NPV) • Internal Rate of Return (IRR) • Payback period • Discounted cash flows • Financial analysis • Capital assets pricing model • Simple interest, Compound interest & annuities
4.40+
65+ Reviews
86+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Suggest a composition for the halogen compound whose mass spectrum is shown in the figure. Assign each of the major peaks. 100 127 173 160 25 158 162 7 48 HT 79 91 160 170 208 35 160 173 IITI'T1 30...
-
Suggest a structure for the compound (4). Using curly arrow notation, draw out the mechanism for the formation of compound (4). Use 3-dimensional structures to rationalize the relative...
-
Suggest a structure for the compound (5). Using curly arrow notation, draw out the mechanism for the conversion of compound (5) into compound (6). Use 3-dimensional structures to rationalize the...
-
What is control resolution in a robot positioning system?
-
1. Explain the pros and cons of the politics- administration dichotomy as espoused by Woodrow Wilson 2. Discuss the various uses of public budget
-
For parts (a)-(e), use the function y=x2 from x=0 to x=1 with n equal subintervals and the function evaluated at the right-hand endpoints. (a) Find a formula for the sum of the areas of the n...
-
What are the common cost-based hotel pricing methods?
-
The following selected transactions occurred during 2012 for Caspian Importers. The company ends its accounting year on April 30, 2012: Feb 1 Loaned $14,000 cash to Brett Dowling on a one-year, 8%...
-
You expect the inflation rate to be 1.5 percent and the U.S. Treasury bill yield to be 1.8 percent for the next year. The risk premium on small-company stocks is 9.2 percent. What rate of return do...
-
A spherical vessel used as a reactor for producing pharmaceuticals has a 10-mm-thick stainless steel wall (k = 17 W/m K) and an inner diameter of I m. The exterior surface of the vessel is exposed...
-
Explain how the peaks at m/z 115, 101, and 73 arise in the mass spectrum of 3-methy-3-heptanol.
-
The mass spectra of 3-ethyl-2-pentanone and 4-methyl-2-pentanone are as follows. Explain which spectrum goes with which compound, what is the structure of the ion responsible for the peak at m/z 43...
-
Massie Corporation has an investment in the common stock of Witzmann Corporation. Massie acquired its investment at Witzmanns book value, so there was no excess cost associated with the investment....
-
A year-end cut-off error occurred in 2017. A large shipment of nonperishable supplies arrived from South America on the last day of 2017 and had been left in the shipping containers outside the main...
-
15. [5] It's not so difficult to incorporate time-varying volatility into the BSM model as long as the time variation is not random. Assume a BSM economy, but this time, assume that the volatility of...
-
3.6. Explain and discuss the potential benefits to be gained by using blade twist, plan- form taper, low solidity, large radius, and low rotational speed for the main rotor of a heavy lift helicopter...
-
2. A VRM (Voltage Regulator Modul) is used to supply the voltageto the CPU of a computer. In the new generation of microprocessors,whose power consumption is 100W, the input voltage to the VRM is12V...
-
Alvarado Company produced 6,400 units of product that required 5.5 standard direct labor hours per unit. The standard variable overhead cost per unit is $5.80 per direct labor hour. The actual...
-
What were the objectives that Carlsberg-Tetley set out to achieve through sponsorship of the England cricket teams?
-
When an electric field is applied to a shallow bath of vegetable oil, why do tiny bits of thread floating in the oil align with the field like compasses in a magnetic field?
-
What did investors in Treasury bonds expect the Federal Reserve to do with interest rates?
-
When two substituents are on the same side of a ring skeleton, they are said to be cis, and when on opposite sides, trans (analogous to use of those terms with 1,2-disubstituted alkene isomers)....
-
Examine the diagram showing an a-helical protein structure in Section 2.13E. Between what specific atoms and of what functional groups are the hydrogen bonds formed that give the molecule its helical...
-
Using a three-dimensional formula, show the direction of the dipole moment of CH3OH. Write (+ and (- signs next to the appropriate atoms.
-
Required information Skip to question [ The following information applies to the questions displayed below. ] Forten Company's current year income statement, comparative balance sheets, and...
-
Give a breakdown of all the intangible assets with the values shown in the statement of financial position of Unilever in 2022.
-
1-The yield to maturity will be greater than the coupon rate when a bond is selling at a premium. Select one: a. False b. True 2-Which one of the following would have the greatest present value,...

Study smarter with the SolutionInn App


