The mass spectra of 3-ethyl-2-pentanone and 4-methyl-2-pentanone are as follows. Explain which spectrum goes with which compound,
Question:
The mass spectra of 3-ethyl-2-pentanone and 4-methyl-2-pentanone are as follows. Explain which spectrum goes with which compound, what is the structure of the ion responsible for the peak at m/z 43 in eachspectrum?
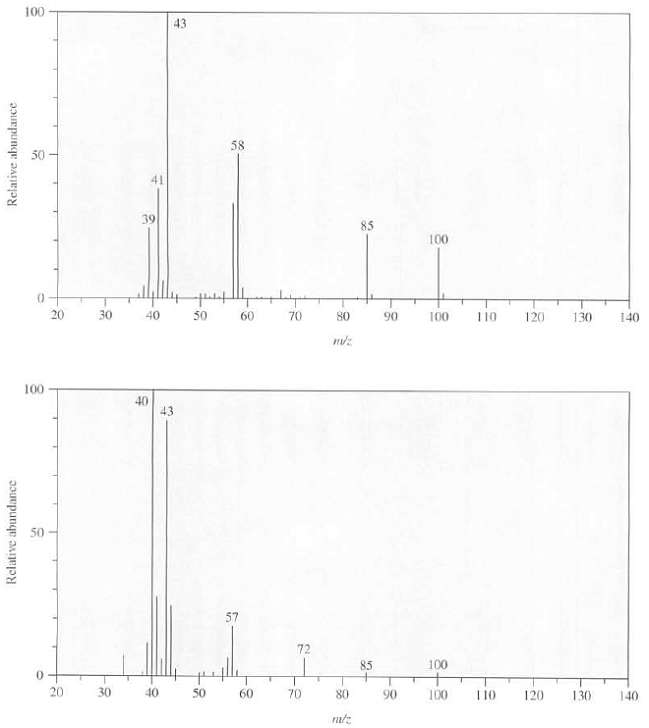
Transcribed Image Text:
100 43 58 50 41 39 85 100 20 30 40 50 70 60 80 90 100 110 120 130 140 100 40 43 57 72 100 85 20 30 40 50 60 70 80 90 100 T10 120 130 140 Relative abundance Relative abundance
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (16 reviews)
Both the ketones produce fragment ions at mz 43 and 85 due t...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
The mass spectra of 1-methoxybutane, 2-methoxybutane, and 2-methoxy-2-methylpropane are shown in Figure 13.7. Match the compounds with the spectra. 100 73 80 S 60 57 20 0 10 20 30 40 50 60 70 80 90...
-
The mass spectra of two very stable cycloalkanes both show a molecular ion peak at m/z = 98. One spectrum shows a base peak at m/z = 69, the other shows a base peak at m/z = 83. Identify the...
-
The mass spectra of acid derivatives follow the principles shown in Chapter 18 for other carbonyl compounds and for alkoxy groups. Both McLafferty rearrangements and alpha-cleavages are common. The...
-
Holly needs $21,800 worth of new equipment for his shop. He can borrow this money at a discount rate of 11% for a year. Find the amount of the loan Holly should ask for so that the proceeds are...
-
IBM has a bond issue outstanding with 14 years to maturity. When originally issued the bond had a par value of $1,000, a stated coupon rate of 12% and 15 years to maturity. Currently, similar risk...
-
The figure gives the times that it takes a Mitsubishi Eclipse GSX to reach speeds from 0 mph to 100 mph, in increments of 10 mph, with a curve connecting them. The area under this curve from t=0...
-
What factors must be considered in setting the final prices of menu items?
-
As Fiona and Rip head out of the room with Andy, Hy realizes the bench has emptied, so he asks you, the fourth team member, to coach Andy on his bar chart by making a list of the problems you see in...
-
The accrual basis, not the cash basis, recognizes all aspects of the credit phenomenon. Select one: True False
-
There is a lottery with n coupons and n people take part in it. Each person picks exactly one coupon. Coupons are numbered consecutively from 1 to n, n being the maximum ticket number. The winner of...
-
Suggest a structure for the compound whose mass spectrum is asfollows: 100 107 81 93 188 70 80 110 120 90 100 130 150 160 170 140 180 190 200 Relative abundance
-
Compounds A and B are isomers with the formula C3H6O. A has a peak at 1730 cm ?1 in its IR spectrum and B has a peak at 1715cm ?1 , the mass spectra of A and B are as follows, show the structures of...
-
On September 1, 2015, Winans Corporation acquired Aumont Enterprises for a cash payment of 700,000. At the time of purchase, Aumonts statement of financial position showed assets of 620,000,...
-
A company must decide between scrapping or reworking units that do not pass inspection. The company has 16,000 defective units that have already cost $132,000 to manufacture. The units can be sold as...
-
according to the phase rule, the triple point of a pure substance is A. invariant B. u nivariant C. bivariant D. none of the above
-
33. If the equipment in the previous question had sold for $15,000, the correct entry would be: a. Cash debit $15,000. Gain credit $3,000. $12,000 Equipment credit b. Cash debit $15,000. Debit a loss...
-
The banks play a central role in financial intermediation in New Zealand. 1.What is financial intermediation? Who performs it? and why is it important? 2.What is Qualitative Asset transformation...
-
Consider the following information attributed to the material management department Budgeted usage of materials - handling labor - hours 3,700 Budgeted cost pools: Fixed costs $166,500 Variable costs...
-
The notion of fit is discussed earlier in the chapter. Is there a fit between Tetley Bitter and English cricket?
-
What kind of rays are X-rays?
-
In late November and early December 2006, there was speculation in the financial markets as to what the Federal Reserve planned to do with interest rates. After raising the federal funds interest...
-
Trichloromethane (CHCl3, also called chloroform) has a larger dipole moment than CFCl3. Use three-dimensional structures and bond moments to explain this fact.
-
Indicate the direction of the important bond moments in each of the following compounds (neglect C-H bonds). You should also give the direction of the net dipole moment for the molecule. If there is...
-
Write structural formulas for all of the alkenes with (a) The formula C2H2Br2 and (b) The formula C2Br2Cl2. In each instance designate compounds that are cis-trans isomers of each other. Predict the...
-
! Required information [ The following information applies to the questions displayed below. ] Year 1 total cash dividends Year 2 total cash dividends Year 3 total cash dividends Year 4 total cash...
-
WISE-HOLLAND CORPORATION On June 15, 2013, Marianne Wise and Dory Holland came to your office for an initial meeting. The primary purpose of the meeting was to discuss Wise-Holland Corporation's tax...
-
! Required information [ The following information applies to the questions displayed below. ] Year 1 total cash dividends Year 2 total cash dividends Year 3 total cash dividends Year 4 total cash...

Study smarter with the SolutionInn App


