Compounds A and B are isomers with the formula C3H6O. A has a peak at 1730 cm
Question:
Compounds A and B are isomers with the formula C3H6O. A has a peak at 1730 cm?1 in its IR spectrum and B has a peak at 1715cm?1, the mass spectra of A and B are as follows, show the structures of A and B.
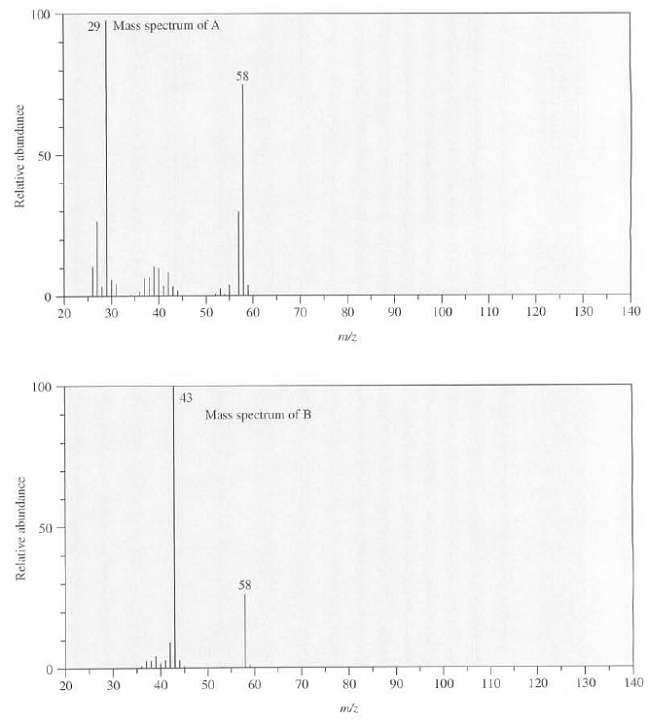
Transcribed Image Text:
100 29 | Mass spectrum of A 58 50 140 70 80 90 100 110 120 130 20 30 40 50 60 100 43 Mass spectrum of B 50 58 140 120 130 70 80 90 100 110 30 40 50 60 20 Relative abundance Relative abundance
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 72% (11 reviews)
The DU for both isomers is 1 Based on their PR spectra this must result from a carbonyl group ...View the full answer

Answered By

Gabriela Rosalía Castro
I have worked with very different types of students, from little kids to bussines men and women. I have thaught at universities, schools, but mostly in private sessions for specialized purpuses. Sometimes I tutored kids that needed help with their classes at school, some others were high school or college students that needed to prepare for an exam to study abroud. Currently I'm teaching bussiness English for people in bussiness positions that want to improve their skills, and preparing and ex-student to pass a standarized test to study in the UK.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Compounds A and B are isomers having the molecular formula C4H8O3. Identify A and B on the basis of their 1H NMR spectra. Compound A: 1.3 ppm (3H, triplet); 3.6 ppm (2H, quartet); 4.1 ppm (2H,...
-
Compounds A and B are isomers of molecular formula C10H14. Identify each one on the basis of the 13C NMR spectra presented in Figure 13.41.
-
Compounds A, B, and C are isomers with the formula C5H11Br. Their broadband proton-decoupled 13C NMR spectra are given in Fig. 9.32. Information from the DEPT 13C NMR spectra is given near each peak....
-
At Blossom Company, events and transactions during 2020 included the following. The tax rate for all items is 20%. (1) Depreciation for 2018 was found to be understated by $148000. (2) A strike by...
-
How does administrative responsibility contribute to the attainment of public interest?
-
Use the formulas given below to answer the following questions 1. Find SL(10) and SR(10). 2. Find SL(100) and SR(100). 3. Find limn( SL and limn( SR. When the area under f (x) = x2 x from x = 0 to x...
-
What is demand-based pricing? How can a foodservice operator find out what the market will bear?
-
Listed below are several terms and phrases associated with earnings per share. Pair each item from List A with the item from List B (by letter) that is most appropriately associated withit. List A...
-
The Riteway Ad Agency provides cars for its sales staff. In the past, the company has always purchased its cars from a dealer and then sold the cars after three years of use. The company's present...
-
Rhetorix, Inc. produces stereo speakers. The selling price per pair of speakers is $1,000. The variable cost of production is $300 and the fixed cost per month is $49,000. For November, the company...
-
The mass spectra of 3-ethyl-2-pentanone and 4-methyl-2-pentanone are as follows. Explain which spectrum goes with which compound, what is the structure of the ion responsible for the peak at m/z 43...
-
Compounds C and D are isomers with the formula C9H12, in addition to other absorption peaks, both compounds show a peak near 7.25 ? (area 5) in their 1H-NMR spectra. Their mass spectra are as follow,...
-
Arcadia Ltd has only one product, a garden gnome, for which it plans to increase production and sales during the first half of next year. The plans for the next eight months are as follows: The...
-
5) A frictionless rod of length L rotates counterclockwise in the with constant angular speed w at an angle a to the z axis. A bead of mass m, free to slide on the rod, leaves the origin with initial...
-
1) Louisa is a corn farmer in Illinois. She anticipates a harvest in August of 3 million bushels of yellow corn. Today is May. Louise plans to hedge her sale of corn in August using corn futures...
-
2. DETAILS MY NOTES In a statistical test, we have a choice of a left-tailed test, a right-tailed test, or a two-tailed test. Is it the null hypothesis or the alternate hypothesis that determines...
-
2. The model of a two-story building shown in Figure 2. The girders are assumed to be rigid, and the columns have flexural rigidities EI and EI2, with negligible masses. The stiffness of each column...
-
Prepare journal entries to record these transactions. (List all debit entries before credit entries. Credit account titles are automatically indented when amount is entered. Do not indent manually....
-
Use the examples above as a basis for a discussion of the legality and morality of ambush marketing. Following this discussion, develop your own definition of ambush marketing.
-
When you weigh yourself on good old terra firma (solid ground), your weight is 142 lb. In an elevator your apparent weight is 121 lb. What are the direction and magnitude of the elevator's...
-
Three economists at the Federal Reserve Bank of St. Louis Andrew Levin, Fabio Nattaluci, and Jeremy Piger have attempted to measure the effects of a short-lived increase in actual inflation on...
-
Write structures for all compounds with molecular formula C4H6O that would not be expected to exhibit infrared absorption in the 3200-3550-cm-1 and 1620-1780-cm-1 regions.
-
Add curved arrows to the following reactions to indicate the flow of electrons for all of the bond-forming and bond-breaking steps. (a) (b) H.
-
Most carboxylic acids dissolve in aqueous solutions of sodium bicarbonate (NaHCO3) because, as carboxylate salts, they are more polar. Write curved arrows showing the reaction between a generic...
-
*please calculate irr in excel
-
Which of the following would not be a period cost? Research and development Direct materials Office supplies Advertising costs
-
\ table [ [ Activity Cost Pool,Activity Measure,Total Cost,Total Activity ] , [ Machining , Machine - hours,$ 3 3 0 , 0 0 0 , 1 5 , 0 0 0 MHs ] , [ Machine setups,Number of setups,$ 3 0 0 , 0 0 0 , 5...

Study smarter with the SolutionInn App


