Tell whether each of the following reactions is an oxidation, a reduction, orneither: (a) CH3CH CH3CH2OH
Question:
Tell whether each of the following reactions is an oxidation, a reduction, orneither:
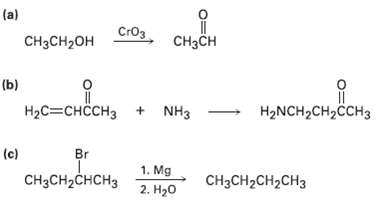
Transcribed Image Text:
(a) Стоз CH3CH CH3CH2OH (b) HаС—снссHз + NHз — НаNCH2CH2CCH3 Br (c) 1. Mg CHзCH2CHCHз CняCH2CH2CH3 2. H20
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 62% (16 reviews)
a This reaction is an oxidation b The reac...View the full answer

Answered By

Arshad Ahmad
Well, I am really new to tutoring but I truly believe a good student can be a better teacher. I have always been a topper at school. I passed my Chartered Accountancy at a very young age of 23, a rare feat for most of the students. I am really dedicated to whatever work I do and I am very strict regarding deadlines. i am always committed and dedicated to whatever work allotted to me and I make sure it is completed well within deadline and also I try to give my best in whatever I do. Hope we will have a good time studying together.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Indicate whether each of the following reactions is an oxidation reaction, a reduction reaction, or neither: a. b. c. d. e. f. H2 CHj 3CI partially deactivatedCH Pd HBr RCH CHRRCH2CHR Br2 H2CrO4...
-
Tell whether each of the following reactions is likely to be SN1, SN2, E1, E1cB, orE2: NaN3 (a) CHCH2CH2H2Br CH3CH2CH2CH,N=N=N THE CI (b) , CCH-CHCH2CH CHCH2CHCHCH3 Ethanol (c) H CI -CH -- -CH (d)...
-
Tell wbether each of the following reactions favors reactants or products at equilibrium. (Assume that all reactants and products are soluble.) (a) CH3CI + I- CH3I + CI- (b) CH3CI + -OCH3 CH3OCH3 +...
-
Scranton Refrigeration Corporation began operations at the beginning of the current year. One of the companys products, a compressor, sells for $370 per unit. Information related to the current years...
-
Refer to the BMW Company case. Design a spreadsheet that will allow the firm's managers to estimate what percentage of the firm's net income 10 years into the future will be devoted to disposal of...
-
The spherical gel capsule shown in the figure at the top of the next column is used for long-term, sustained drug release. A saturated liquid solution containing the dissolved drug (solute A) is...
-
Todd and Carlo became partners on January 1, 2005, in their joint venture ToLo Sports Bar. Todd put in $50,000, while Carlos share was $75,000. The net income for the year is $12,000. If the income...
-
Using the SoakNFun Swim Park information presented, do the following tasks. SoakNFun Swim Park sells individual and family tickets. With a ticket, each person receives a meal, three beverages, and...
-
Walt Disney Company's Sleeping Beauty Bonds In July 1993, the Walt Disney Company issued $300,000,000 in senior debentures (bonds). The debentures carried an interest rate of 7.55%, payable...
-
MGM International operates casinos and resorts across the U.S. and in China. The company reported the following in its SEC filings. We maintain an allowance for doubtful casino accounts at all of our...
-
Which of the following compounds have the same oxidation level, and which have differentlevels? OH 5.
-
How would you carry out the followingsyntheses? Cyclohexene Cyclohexanol Cyclohexane ~/~/al
-
My good grades are a result of the fact that the number of hours I study each week put me in the 90th percentile for study time. Does It Make Sense? For Exercises, determine whether the statement...
-
Could I obtain assistance with these . problems? 1. Find the coordinates of the turning points of the curve y=3x^4-8x^3-30x^2+72x+5. Determine the nature of these points. "Determine the nature"...
-
1 . In 1 9 6 0 the homeownership rate in the United States was 6 2 % . Is there evidence to indicate that the homeownership rate is now higher? To answer the question, the researchers sample 5 0 2...
-
A certain disease is classified into 4 stages that distinguish how developed the disease is. Researchers studying a new potential treatment recruited over 100 patients with varying stages of the...
-
1. (20) Let and Dor {abnm or 2n m} = Dand = {a"b" nm and 2n m}. Prove that Dor and Dand are both context-free.
-
Given n samples 1 , 2 , . . . , x 1 ,x 2 ,...,x N drawn independently from a Poisson distribution unknown parameter , find the MLE of . = = 1 MLE = i=1 n x i = = 1 MLE =n i=1 n x i = = 1 MLE = i=1 n...
-
Outline circumstances that call for verbal, written, and electronic communication methods.
-
Use critical values to test the null hypothesis H0: 1 2 = 20 versus the alternative hypothesis H0: 1 2 20 by setting a equal to .10, .05, .01, and .001. How much evidence is there that the...
-
The heat of atomization is the heat required to convert a molecule in the gas phase into its constituent atoms in the gas phase. The heat of atomization is used to calculate average bond energies....
-
Draw Lewis structures for the following free radicals. (a) The ethyl radical, (b) The tert-butyl radical, (CH3)3C (c) The isopropyl radical (2-propyl radical) (d) The iodine atom CH3 CH2
-
(a) Using bond-dissociation enthalpies from Table 4-2 (page 143), calculate the heat of reaction for each step in the free-radical bromination of methane. (b) Calculate the overall heat of reaction....
-
The reaction of tert-butyl chloride with methanol Is found to follow the rate equation Rate = kf [(CH3)3C-Cl] (a) What is the kinetic order with respect to tert-butyl chloride? (b) What is the...
-
1. (A nice inharitage) Suppose $1 were invested in 1776 at 3.3% interest compounded yearly a) Approximatelly how much would that investment be worth today: $1,000, $10,000, $100,000, or $1,000,000?...
-
Why Should not the government subsidize home buyers who make less than $120K per year. please explain this statement
-
Entries for equity investments: 20%50% ownership On January 6, 20Y8, Bulldog Co. purchased 25% of the outstanding common stock of $159,000. Gator Co. paid total dividends of $20,700 to all...

Study smarter with the SolutionInn App


