Tell whether each of the following reactions is likely to be SN1, SN2, E1, E1cB, orE2: NaN3
Question:
Tell whether each of the following reactions is likely to be SN1, SN2, E1, E1cB, orE2:
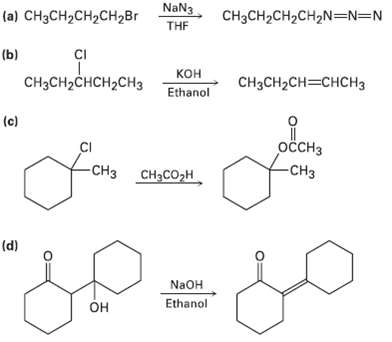
Transcribed Image Text:
NaN3 (a) CHзCH2CH2сH2Br CH3CH2CH2CH,N=N=N THE CI (b) ононалонен, кон CнзCH-CHCH2CHЗ CHзCH2CH—CHCH3 Ethanol (c) оссHз CI -CHз сн-со-н -CHз (d) NaOH Ethanol Он
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (14 reviews)
a CH3CHCHCHBr NaN3 primary The reaction occurs by an SN2 mechani...View the full answer

Answered By

Muhammad adeel
I am a professional Process/Mechanical engineer having a vast 7 years experience in process industry as well as in academic studies as a instructor. Also equipped with Nebosh IGC and lead auditor (certified).
Having worked at top notch engineering firms, i possess abilities such as designing process equipment, maintaining data sheets, working on projects, technical biddings, designing PFD and PID's etc.
Having worked as an instructor in different engineering institutes and have been involved in different engineering resrearch projects such as refinery equipment designing, thermodynamics, fluid dynamics, chemistry, rotary equipment etc
I can assure a good job within your budget and time deadline
4.90+
52+ Reviews
60+ Question Solved
Related Book For 

Question Posted:
Related Video
In this video, A mixture of methanol and air in a large polycarbonate bottle is ignited. The resulting rapid combustion reaction, often accompanied by a dramatic ‘whoosh’ sound and flames, demonstrates the large amount of chemical energy released in the combustion of alcohol
Students also viewed these Organic Chemistry questions
-
Tell whether each of the following reactions is an oxidation, a reduction, orneither. (a) NABH4 H20 CH;CH- CH3CH2CH2OH (b) OH 1. BH3 2. NaOH, H202
-
Tell whether each of the following reactions is an oxidation, a reduction, orneither: (a) CH3CH CH3CH2OH (b) HH + NH NCH2CH2CCH3 Br (c) 1. Mg CHCH2CHCH CCH2CH2CH3 2. H20
-
Tell whether each of the following molecules has a meso stereoisomer. (a) (b) CH CHCH CHCH Cl CH, CHCH2CH,CH CI
-
All individuals in Canada have the responsibility to take meaningful actions towards truth and reconciliation. As business professionals, we take it a step further and talk about our responsibility...
-
What some of the benefits that exist for employees who learn English
-
Coach has been described as a textbook lesson on how to revitalize a brand. The same could be said for Burberry, the British fashion goods company discussed in Chapter 1. Locate some articles about...
-
Because your organization is not a cost leader, does this system need to be cost feasible? Explain why or why not.
-
In the manufacture of nitric acid, ammonia and preheated air are mixed to form a gas containing 10.0 mole% NH3 at 600C. The ammonia is then catalytically oxidized to form NO2, which is absorbed in...
-
Homework: Chapter 9 Homework Save Score: 0 of 2 pts 6 of 10 (2 complete) HW Score: 20%, 4 of 20 pts S9-12 (similar to) Question Help Stampede Corporation is preparing its cash payments budget for...
-
Two years ago, you represented Mr. Smith in setting up a close corporation for his business and for certain personal investments. That work has long since been completed, and you have not represented...
-
Which isomer would you expect to undergo E2 elimination faster, trans1-bromo-4-tert-butylcyclohexane or cis-1-bromo-4-tert-butylcyclohexane? Draw each molecule in its more stable chair conformation,...
-
Write the product you would expect from reaction of each of the following alkyl halides with (i) Na + ? SCH3 and (ii) Na + ?? OH (yellow-green = Cl): (a) (c) (b)
-
Among the elderly, is there a relationship between education and health? Using the PewHealth dataset, select cases so that you are looking only at those aged 80 and older. Assess the relationship...
-
The following information about the payroll for the week ended December 30 was obtained from the records of Saine Co.: Salaries: Sales salaries Deductions: $180,000 Income tax withheld $65,296...
-
You have just been hired as the chief executive officer (CEO) in a medium-sized organization. The organization is not suffering financially, but neither is it doing as well as it could do. This is...
-
The following is the selling price and cost information about three joint products: X Y Z Anticipated production 1 2 , 0 0 0 lbs . 8 , 0 0 0 lbs . 7 , 0 0 0 lbs . Selling price / lb . at split - off...
-
calculate the maximum bending compressive stress of the following section under NEGATIVE bending moment of 216KN.m. 216mm 416mm 316mm 115mm
-
Need assistance with the following forms: 1040 Schedule 1 Schedule 2 Schedule C Schedule SE Form 4562 Form 8995 Appendix B, CP B-3 Christian Everland (SS number 412-34-5670) is single and resides at...
-
6 2 A- Walters, a trader, uses vans for making deliveries. The following is an extract from his balance sheet as at 31 December 20X0: 23,040 5,760 17,280 On 7 March 20X1 Walters purchased, for cash,...
-
Answer the following two independent questions. a. MM Corporation is considering several proposed investments for the coming budget year. MM produces electrical apparatus for industrial complexes....
-
Consider the reaction: A reaction mixture initially contains a CO partial pressure of 1344 torr and a H 2 O partial pressure of 1766 torr at 2000 K. Calculate the equilibrium partial pressures of...
-
Arrange each group of compounds in order of increasing acidity. (a) Phenol, ethanol, acetic acid (b) P-toluenesulfonic acid, acetic acid, chloroacetic acid (c) Benzoic acid, o-nitrobenzoic acid,...
-
What do the following pKa values tell you about the electron-withdrawing abilities of nitro, cyano, chloro, and hydroxyl groups? CH COOH CH,COOH CH COOH CHACOOH CHACOOH NO, 1.68 CN 2.46 CI 2.86 3.83...
-
Given the structure of ascorbic acid (vitamin C): (a) Is ascorbic acid a carboxylic acid? (b) Compare the acid strength of ascorbic acid (pKa = 4.71) with acetic acid. (c) Predict which proton in...
-
Given the following financial data for the Smith Corporation, calculate the length of the firm's operating cycle (OC). Sales $2,610,000 Cost of Good Sold $2,088,000 Inventory $ 278,400 Accounts...
-
The predetermined overhead rate is usually calculated Group of answer choices At the end of each year At the beginning of each month At the beginning of the year At the end of the month
-
ajax county collects property taxes for the cities within the county, Ajax county collected 1000 from citizens in Beatty city that belong to Beatty city what would be the appropriate entries for ajax...

Study smarter with the SolutionInn App


