Consider the reaction: A reaction mixture initially contains a CO partial pressure of 1344 torr and a
Question:
Consider the reaction:
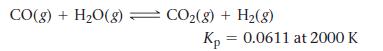
A reaction mixture initially contains a CO partial pressure of 1344 torr and a H2O partial pressure of 1766 torr at 2000 K. Calculate the equilibrium partial pressures of each of the products.
Transcribed Image Text:
CO(g) + H₂O(g) CO₂(g) + H₂(g) Kp = 0.0611 at 2000 K
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)
Solution The equilibrium partial pressures of the products can be cal...View the full answer

Answered By

Hillary Waliaulah
As a tutor, I am that experienced with over 5 years. With this, I am capable of handling a variety of subjects.
5.00+
17+ Reviews
30+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Consider the reaction: a. A reaction mixture at 175 K initially contains 522 torr of NO and 421 torr of O 2 . At equilibrium, the total pressure in the reaction mixture is 748 torr. Calculate K p at...
-
Consider the reaction: A reaction mixture initially contains a Br 2 partial pressure of 755 torr and a Cl 2 partial pressure of 735 torr at 150 K. Calculate the equilibrium partial pressure of BrCl....
-
You have been assigned the task of measuring the equilibrium constant for the reaction N 2 O 4 2NO 2 as a function of temperature. To do so, you evacuate a rigid 2-liter vessel equipped with a...
-
1. As shown by point D in Fig 3.1, the volume of an ideal diatomic gas is 2.00L at standard condition (STP, T=273.15K, P=101.3kPa). The gas is heated to A with its volume conserved, expands...
-
Explain why profit-linked productivity measurement is important.
-
Under what circumstances can a corporation be held legally responsible for a white-collar offense committed by one of its agents or employees?
-
Conlon Travel Supplies entered into the following transactions during 1996. 1. Purchased inventory on account. 2. Purchased plant machinery by issuing long-term debt. 3. Made a principal payment on...
-
What are the ethical issues involved in the Madoff case? The fraud perpetrated by Bernard Madoff that was discovered in December 2008 was what is known as a Ponzi scheme. A Ponzi scheme works...
-
Price, Variable Cost per Unit, Contribution Margin, Contribution Margin Ratio, Fixed Expense For each of the following independent situations, calculate the amount(s) required. Required: 1. At the...
-
Dr. Jordan Davis has hired your professional services to file her income tax return. Dr. Davis is a retired surgeon. Due to her failing eyesight, Jordan was required to retire from her occupation at...
-
Consider the reaction: Find the equilibrium partial pressures of A and B for each value of K p . Assume that the initial partial pressure of B in each case is 1.0 atm and that the initial partial...
-
Consider the reaction: If a reaction mixture initially contains 0.175 M SO 2 Cl 2 , what is the equilibrium concentration of Cl 2 at 227 C? SOCl(g) = SO(g) + Cl(g) K 2.99 x 10-7 at 227 C =
-
Ulterior Motives You are driving a classic 1954 Nash Ambassador with a friend who is sitting to your right on the passenger side of the front seat. The Ambassador has flat bench seats. You would like...
-
What is the most important take-home point that you learned from this video? https://www.youtube.com/watch?v=nUZqvsF_Wt0 2. Policy Problems. What is onepolicy that creates inequality in the labor...
-
An employee had $20,300 in gross earnings up to September 20, 2021. She has the following information for her pay for the week ending September 27, 2021. Her employer contributes 100% toward CPP and...
-
If the dose rate from a sample of Ga-67 is 0.052 mSv per hour at a distance of 1.1 m, then what would be dose rate at 3.5 m ?
-
A 1.6x10^9 p/s point source of Po210-Be source of 4.5 MeV is stored behind a X cm of paraffin, the dose equivalent rate is not to exceed 0.10 mSvh-1h at a distance of 1m. What is the X cm needed to...
-
X 10 Let A = -9 y 7 4 Z 210 If the kernel of A contains the vector what are x, y, and z? -2
-
Forward Par Swap Rate yn,N(t) is defined Pn+1,N(t) is called the present value of a basis point (PVBP). A swaption gives the holder the right not the obligation to enter into a particular swap...
-
Which internal control principle is especially diffi cult for small organizations to implement? Why?
-
For the system shown in Fig. 8.17, compute the power delivered by the pump to the water to pump 50 gal/min of water at 60F to the tank. The air in the tank is at 40 psig. Consider the friction loss...
-
Fuel oil (sg = 0.94) is being delivered to a furnace at a rate of 60 gal/min through a 1 -in Schedule 40 steel pipe. Compute the pressure difference between two points 40.0 ft apart if the pipe is...
-
Figure 8.18 shows a system used to spray polluted water into the air to increase the waters oxygen content and to cause volatile solvents in the water to vaporize. The pressure at point B just ahead...
-
You just won a stock picking contest that will pay you $24,000 a year for 26 years, and you get the first payment today. What is the prize worth to you today if your annual opportunity cost rate is...
-
a) Discuss in about 200 words the Hierarchy of the information- based business decision makers. Which level has the best chance of success and why? b) Differentiate between deductive reasoning and...
-
Adelphi Company has budgeted activity for March to reflect net income $115,000. All sales are credit sales. Receivables are planned to increase (decrease -) by $21,000 payables to increase (decrease...

Study smarter with the SolutionInn App


