Terminal alkynes react with Br2 and water to yield bromo ketones. For example: Propose a mechanism for
Question:
Terminal alkynes react with Br2 and water to yield bromo ketones. For example: Propose a mechanism for the reaction. To what reaction of alkenes is the processanalogous?
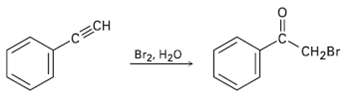
Transcribed Image Text:
-CECH Br2, H20 CH2Br
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 56% (16 reviews)
This reaction mechanism is similar to the mechan...View the full answer

Answered By

Hillary Waliaulah
As a tutor, I am that experienced with over 5 years. With this, I am capable of handling a variety of subjects.
5.00+
17+ Reviews
30+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Propose a mechanism for the following reaction: CH3 CH CH H,504. H,C
-
Propose a mechanism for the following reaction: CH CH, C-CH, CH2OH Cl CH3
-
Propose a mechanism for the following reaction: CI Alcl
-
What are some techniques of good writers? Which ones do you use regularly?
-
A construction contractor is responsible for a project with seven key tasks. Some of the tasks can begin at any time, but others have predecessor tasks that must be completed previously. The...
-
What pressure drop per foot of tube is caused by the shear stress in Problem 7.17? Data From Problem 7.17 For water flowing in a 0.1-in.-diameter tube, the velocity distribution is parabolic (see...
-
Acme Hydraulic Press Co. manufactured large presses and sold them throughout the United States. The agreement-of-sale contract that Acme executed with its customers specified that they could make no...
-
Gosnell Company produces two products: squares and circles. The projected income for the coming year, segmented by product line, follows: The selling prices are $30 for squares and $50 for circles....
-
Ahmad bought a share ten months ago for $25 a share, got a $3.5 dividend per share last month, and sold the stock today for $21.5 per share. Ahmad falls in a marginal tax bracket of 30%. The tax rate...
-
What do you know about COASE theorem? Under what conditions COASE theorem would not work effectively or break down?
-
Erythrogenic acid, C 18 H 26 O 2 , is an acetylenic fatty acid that turns a vivid red on exposure to light. On catalytic hydrogenation over a palladium catalyst, 5 equivalents of H 2 is absorbed, and...
-
A cumulene is a compound with three adjacent double bonds. Draw an orbital picture of a cumulene. What kind of hybridization do the two central carbon atoms have? What is the geometric relationship...
-
Capital Expenditures, Depreciation, and Disposal Wagner Company purchased a retail shopping center on January 1, 2009, at a cost of $612,000. Wagner estimated that its life would be 25 years and its...
-
Civil What are the challenges of using intermediate structures in the analysis of Pauli structures?
-
Civil What are the advantages of intermediate structures in the analysis of Laguerre structures?
-
Civil What are the disadvantages of using intermediate structures in the analysis of geothermal energy environmental product promotion?
-
Civil How can intermediate structures be used in the analysis of geothermal energy environmental product pulmonary toxins?
-
Civil What challenges arise when using intermediate structures in the analysis of structural robustness?
-
Special event planners and managers have filled a need that was first introduced at hotels and convention centers. They are responsible for planning the event, from start to finish. LO.1
-
For each of the following transactions, indicate whether it increases, decreases, or has no effect on the following financial ratios: current ratio, debt-to-equity ratio, profit margin ratio, and...
-
What reactant types give rise to gas-evolution reactions?
-
Predict the hybridization, geometry, and bond angles for the central atoms in: (a) But-2-ene, CH3CH=CHCH3 (b) CH3CH=NH
-
Predict the hybridization and geometry of the carbon and nitrogen atoms in the following ions. (a) (b) (c) H,N- CH CH CH CH2-CN
-
Draw orbital pictures of the pi bonding in the following compounds: (a) CH3COCH3 (b) HCN (c) CH2=CH-CHCHCN (d) CH3CCCHO (e) CH3CH=CHCH3
-
You want to invest annual amounts over the next 15 years. If your goal is to have $15,000 at the end of that time and if you can earn 8 percent on your invested funds, how much do you need to invest...
-
please explain thoroughly how to do in excel 1. Find the number of units to ship from each factory to each customer that minimizes total cost
-
For esch of the following Independent tranactiona, determine the minimum amount of net income or loas for tox purposes snd the tsxpsyer to which it applies. 1 An individual purchases a $ 1 0 , 0 0 0...

Study smarter with the SolutionInn App


